Indications
Peripheral Artery Disease (PAD) After Revascularization (see Peripheral Artery Disease)
FDA approved 2021 expanded peripheral artery disease (PAD) Indication for Xarelto® (rivaroxaban) plus aspirin to include patients after lower-extremity revascularization (LER) due to symptomatic PAD. News release. Janssen Pharmaceuticals, Inc. Accessed August 24, 2021. https://www.prnewswire.com/news-releases/fda-approves-expanded-peripheral-artery-disease-pad-indication-for-xarelto-rivaroxaban-plus-aspirin-to-include-patients-after-lower-extremity-revascularization-ler-due-to-symptomatic-pad-301361537.html.
Systemic Embolism Prevention in Non-Valvular Atrial Fibrillation (see Atrial Fibrillation)
Clinical Efficacy-General
- FDA Approved in November, 2011 for Stroke Prevention in Non-valvular Atrial Fibrillation
- Systematic Review of Novel Oral Anticoagulants, As Compared to Coumadin (Ann Intern Med, 2012) [MEDLINE]: included chronic non-valvular atrial fibrillation trials (RE-LY Trial (2009): dabigatran, ARISTOTLE Trial (2011): apixaban, ROCKET AF Trial (2011): rivaroxaban) and venous thromboembolism trials (EINSTEIN-DVT (2010): rivaroxaban, RE-COVER (2009): dabigatran, EINSTEIN-PE (2012): rivaroxaban)
- Novel Oral Anticoagulants Decreased All-Cause Mortality, as Compared to Coumadin: risk ratio [RR], 0.88 [95% CI, 0.82 to 0.96]
- Novel Oral Anticoagulants are a Viable Option for Patients Requiring Long-Term Anticoagulation, Although the Treatment Benefits Compared with Coumadin are Small and Vary Depending on the Control Achieved by Coumadin Treatment
- Systematic Review and Meta-Analysis of Bleeding Complications with DOAC’s in Atrial Fibrillation and Venous Thromboembolism (Blood. 2014) [MEDLINE]
- DOAC’s were Associated with Less Major Bleeding Less Fatal Bleeding, Less Intracranial Bleeding, Less Clinically Relevant Bleeding, and Less Total Bleeding, as Compared to Coumadin
- Systematic Review and Meta-Analysis of Mortality Outcomes of DOAC’s in Patients with Atrial Fibrillation and Venous Thromboembolism (J Thromb Haemost, 2015) [MEDLINE]
- DOAC’s were Associated with a Lower Rate of Fatal Bleeding, Lower Case-Fatality Rate of Major Bleeding, Decreased Cardiovascular Mortality, and Decreased All-Cause Mortality, as Compared to Coumadin
Clinical Efficacy-Cost Effectiveness
- Systematic Review of Cost-Effectiveness of Novel Oral Anticoagulants for Stroke Prevention in Non-Valvular Atrial Fibrillation (Rev Port Cardiol, 2015) [MEDLINE]
- Novel Oral Anticoagulants are Cost-Effective for Stroke Prevention in Atrial Fibrillation, as Compared to Coumadin
- Review of Cost-Effectiveness of Novel Oral Anticoagulants for Stroke Prevention in Non-Valvular Atrial Fibrillation (Curr Cardiol Rep, 2015) [MEDLINE]
- Novel Oral Anticoagulants are Cost-Effective for Stroke Prevention in Atrial Fibrillation, as Compared to Coumadin
Venous Thromboembolism (see Deep Venous Thrombosis and Acute Pulmonary Embolism)
Clinical Efficacy
- FDA Approved in November, 2012 for the Treatment of Venous Thromboembolism
- Systematic Review of Novel Oral Anticoagulants, As Compared to Coumadin (Ann Intern Med, 2012) [MEDLINE]: included chronic non-valvular atrial fibrillation trials (RE-LY Trial (2009): dabigatran, ARISTOTLE Trial (2011): apixaban, ROCKET AF Trial (2011): rivaroxaban) and venous thromboembolism trials (EINSTEIN-DVT (2010): rivaroxaban, RE-COVER (2009): dabigatran, EINSTEIN-PE (2012): rivaroxaban)
- Novel Oral Anticoagulants Decreased All-Cause Mortality, as Compared to Coumadin: risk ratio [RR], 0.88 [95% CI, 0.82 to 0.96]
- Novel Oral Anticoagulants are a Viable Option for Patients Requiring Long-Term Anticoagulation, Although the Treatment Benefits Compared with Coumadin are Small and Vary Depending on the Control Achieved by Coumadin Treatment
- Cochrane Systematic Review and Meta-Analysis of DOAC’s (Dabigatran, Rivaroxaban, Apixaban, and Edoxaban) in the Treatment of Acute Symptomatic Venous Thromboembolism (J Thromb Haemost, 2014) [MEDLINE]
- DOAC’s Have Comparable Efficacy to Coumadin and are Associated with a Significantly Lower Risk of Hemorrhagic Complications (Although the Number Needed to Treatment to Prevent One Major Bleed was Notably High at 149)
- Systematic Review and Meta-Analysis of Bleeding Complications with DOAC’s in Atrial Fibrillation and Venous Thromboembolism (Blood. 2014) [MEDLINE]
- DOAC’s were Associated with Less Major Bleeding Less Fatal Bleeding, Less Intracranial Bleeding, Less Clinically Relevant Bleeding, and Less Total Bleeding, as Compared to Coumadin
- Systematic Review and Meta-Analysis of Mortality Outcomes of DOAC’s in Patients with Atrial Fibrillation and Venous Thromboembolism (J Thromb Haemost, 2015) [MEDLINE]
- DOAC’s were Associated with a Lower Rate of Fatal Bleeding, Lower Case-Fatality Rate of Major Bleeding, Decreased Cardiovascular Mortality, and Decreased All-Cause Mortality, as Compared to Coumadin
Venous Thromboembolism Prophylaxis in Medical Patients (see Deep Venous Thrombosis and Acute Pulmonary Embolism)
Clinical Efficacy
- MAGELLAN Non-Inferiority Trial Comparing Rivaroxaban (x 35 +/- 4 Days) to Enoxaparin (x 10 +/- 4 Days) for DVT Prophylaxis in Acutely Ill Medical Patients (NEJM, 2013) [MEDLINE]: multi-center, randomized ( n = 8101)
- At Day 10: Rivaroxaban was Equivalent (2.7%) to Enoxaparin (2.7%), in Terms of Venous Thromboembolism
- At Day 35: Rivaroxaban was Superior (4.4%) to Enoxaparin (5.7%), in Terms of Venous Thromboembolism
- At Day 10: Rivaroxaban Had Significantly Higher Bleeding Risk (2.8%) vs Enoxaparin (1.2%)
- At Day 35: Rivaroxaban Had Significantly Higher Bleeding Risk (4.1%) vs Enoxaparin (1.7%)
- MARINER Trial of Prophylactic Rivaroxaban Begun and Continuing After Hospital Discharge (x 45 Days) in High-Risk Medical Patients (Thromb Haemost, 2016) [MEDLINE]: randomized, double-blind, placebo-controlled
- In Process: endpoints of symptomatic VTE and VTE-related death
Venous Thromboembolism Prophylaxis Post-Knee and Hip Replacement (see Deep Venous Thrombosis and Acute Pulmonary Embolism)
Clinical Efficacy
- FDA Approval: July, 2011 for DVT prophylaxis in hip/knee replacement
- Regulation of Coagulation in Major Orthopedic Surgery Reducing the Risk of DVT and PE (RECORD) Trial (J Bone Joint Surg, 2009) [MEDLINE]
- Rivaroxaban Started 6-8 hrs After Surgery was More Effective than Enoxaparin Started the Previous Evening in Preventing Symptomatic Venous Thromboembolism and All-Cause Mortality, Without Increasing Major Hemorrhage
- Systematic Review Comparing Novel Oral and Other Anticoagulants (Fondaparinux, Dabigatran, Rivaroxaban, Apixaban) to Enoxaparin Used as Venous Thromboembolism Prophylaxis After Major Orthopedic Surgery (Ann Vasc Surg, 2013) [MEDLINE]
- Novel Anticoagulants Can Be Considered as Alternatives to Enoxaparin, Depending on Their Individual Clinical Characteristics and Cost-Effectiveness
- Primary Efficacy (Any DVT, Non-Fatal PE, or All-Cause Mortality) Favored Fondaparinux and Rivaroxaban Over Enoxaparin
- Compared to Enoxaparin, the Bleeding Risk was Similar for All Agents, Except Fondaparinux (Which Manifested a Significantly Higher Any-Bleeding Risk) and Apixaban (Which Manifested a Lower Any-Bleeding Risk)
Pharmacology
Mechanism of Action
- Rivaroxaban is a Selective and Competitive Factor Xa Inhibitor (see Factor Xa Inhibitors)
Metabolism
- Hepatic: CYP3A4 and CYP2J2 enzymes
- Renal: 66% is excreted by kidneys (36% unchanged)
- Feces: 28% is excreted in feces (7% unchanged)
Pharmacokinetics
- Time to Peak Level in Plasma: 2-4 hrs
- Half-Life: 5-9 hrs (11-13 hrs in elderly patients)
Administration
PO Dosing
- Non-Valvular Atrial Fibrillation: 20 mg qday
- Deep Venous Thrombosis (DVT) Prophylaxis for Hip Replacement: 10 mg qday x 35 days (start 6-10 hrs post-op, once hemostasis has been achieved)
- Deep Venous Thrombosis (DVT) Prophylaxis for Knee Replacement: 10 mg qday x 12 days (start 6-10 hrs post-op, once hemostasis has been achieved)
- Venous Thromboembolism (Acute DVT or PE): 15 mg BID x 21 days, then 20 mg qday
- Note: rivaroxaban/apixaban do not require initial parenteral (heparin, etc) anticoagulation for the treatment of venous thromboembolism (see Apixaban)
Effect on Anticoagulation Tests
- Prothrombin Time (PT)/International Normalized Ratio (INR) (see Prothrombin Time): no effect-prolonged (dose-dependent)
- Partial Thromboplastin Time (PTT) (see Partial Thromboplastin Time): no effect-prolonged (dose-dependent, although to a lesser extent than for the INR)
- Thrombin Time (TT) (see Thrombin Time): no effect
- Anti-Factor Xa Activity (see Anti-Factor Xa Activity): no effect-prolonged
- Effect depends on whether the specific laboratory’s activity assay is calibrated for the specific anticoagulant
- Heparin Clotting Time: prolonged
- Bleeding Time : no effect
- Platelet Aggregation: no effect
Hepatic Dose Adjustment
- Child-Pugh Class B or C: avoid use
- Coagulopathy Associated with Liver Disease: avoid use
Renal Dose Adjustment
Treatment of Venous Thromboembolism
- CrCl ≥30 mL/min (US Package Labeing): no dose adjustment necessary
- CrCl 30-50 mL/min (Per Beers Criteria for Patients ≥65 y/o): dose adjustment
- CrCl <30 mL/min (US Package Labeling): avoid use
- ESRD Requiring Hemodialysis: avoid use
Treatment of Non-Valvular Atrial Fibrillation
- CrCl >50 mL/min mL/min: no dose adjustment necessary
- CrCl 15-50 mL/min (US Package Labeling): 15 mg PO qday
- CrCl 30-50 mL/min (Per Beers Criteria for Patients ≥65 y/o): dose adjustment
- CrCl <15 mL/min (US Package Labeling): avoid use
- CrCl <30 mL/min (Per Beers Criteria for Patients ≥65 y/o): avoid use
- ESRD Requiring Hemodialysis: avoid use
Venous Thromboembolism Prophylaxis
- CrCl >50 mL/min: no dose adjustment necessary
- CrCl 30-50 mL/min: no dose adjustment specified, but use with caution
- CrCl 30-50 mL/min (Per Beers Criteria for Patients ≥65 y/o): dose adjustment
- CrCl <30 mL/min: avoid use
- ESRD Requiring Hemodialysis: avoid use
Dose Adjustment for Obesity (2016 International Society of Thrombosis and Hemostasis Recommendations) (J Thromb Haemost, 2016) [MEDLINE]
- BMI ≤40 kg/m2 and Weight <120 kg: standard dosing is recommended for both venous thromboembolism and atrial fibrillation
- BMI >40 kg/m2 or Weight >120 kg: direct oral anticoagulants are not recommended (due to limited clinical data and possibility that patient may be underdosed)
- If Direct Oral Anticoagulants are Used in this Patient Group, Drug-Specific Peak and Trough Levels (Anti-Factor Xa for Apixaban/Edoxaban/Rivaroxaban, Ecarin Time or Dilute Thrombin Time with Appropriate Calibrators for Dabigatran, or Mass Spectrometry for Any of the Agents) Should Be Measured: if levels fall below the expected range, change to coumadin is recommended (rather than dose adjustment of the direct oral anticoagulant)
Drug Interactions
- Azole Anti-Fungals (see Azole Anti-Fungals)
- HIV Protease Inhibitors (see Anti-Retrovirals)
Pregnancy (see Pregnancy)
- Not Approved for Use in Pregnancy: due to possible teratogenicity and secretion in breast milk
Conversion from/to Other Anticoagulants
Conversion From Rivaroxaban
- Conversion From Rivaroxaban -> Unfractionated Heparin/Low Molecular Weight Heparin: discontinue rivaroxaban and start unfractionated heparin drip/low molecular weight heparin at the time the next dose of rivaroxaban would have been taken
Conversion to Rivaroxaban
- Conversion From Coumadin -> Rivaroxaban: start rivaroxaban when INR <3
- Conversion From Low Molecular Weight Heparin -> Rivaroxaban: start rivaroxaban 0-2 hrs prior to the next scheduled evening administration of low molecular weight heparin
- Conversion From Unfractionated Heparin -> Rivaroxaban: stop the heparin drip and start rivaroxaban at the same time
Periprocedural/Perioperative Management of Rivaroxaban Anticoagulation
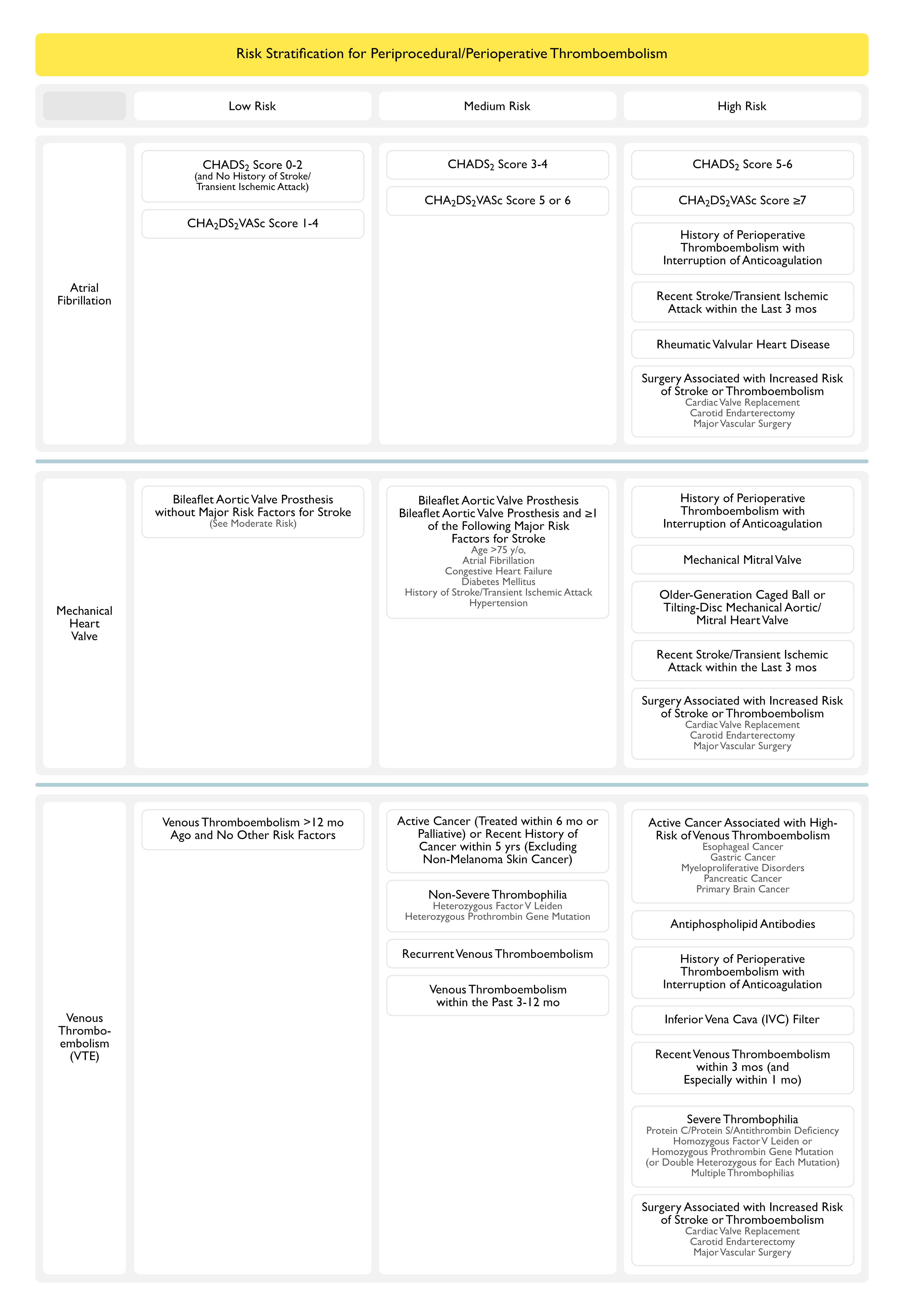
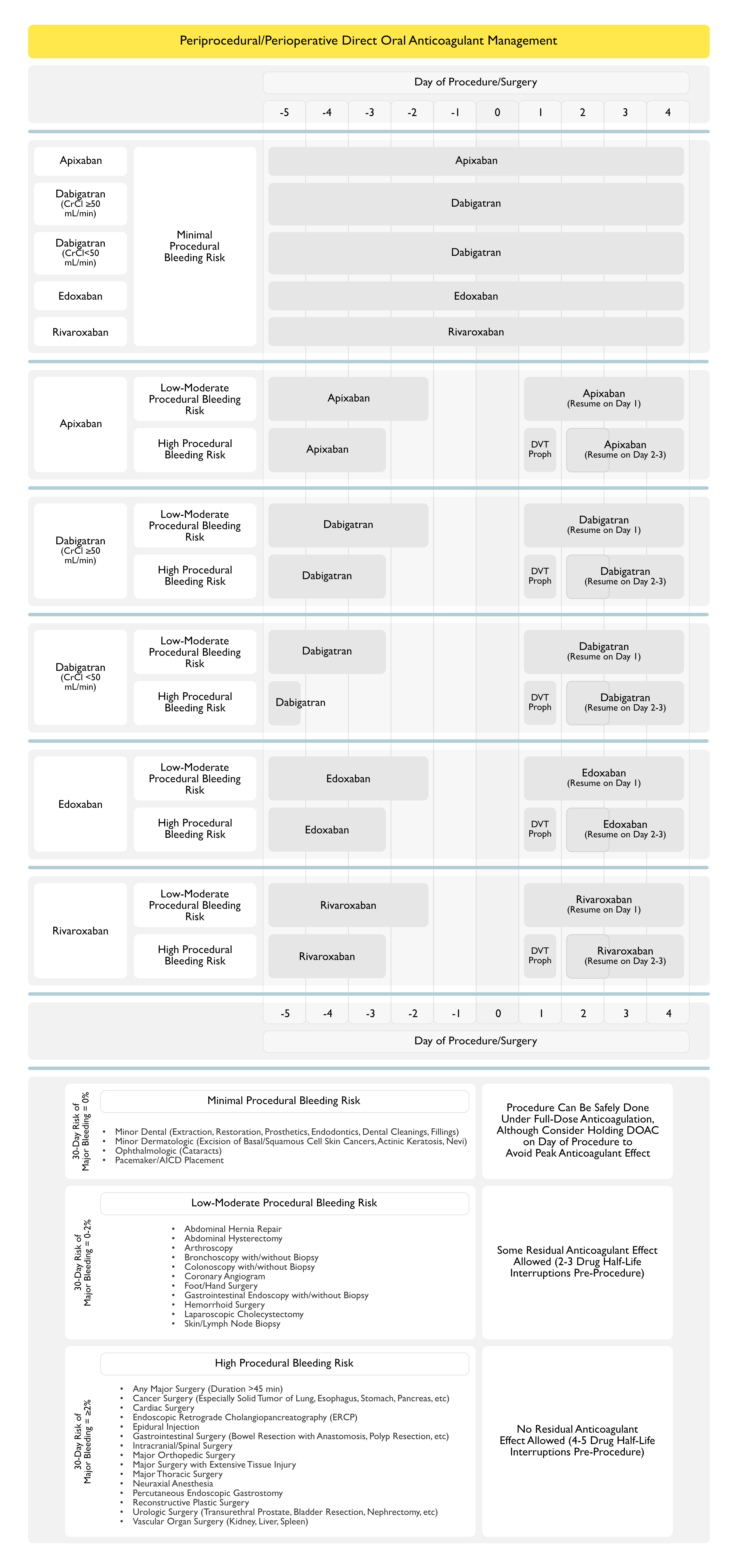
Recommendations for Periprocedural/Perioperative Management of Rivaroxaban (American College of Chest Physicians Clinical Practice Guideline for the Perioperative Management of Antithrombotic Therapy) (Chest, 2022) [MEDLINE]
- In Patients Receiving Rivaroxaban Who Require an Elective Procedure/Surgery, Stop Rivaroxaban 1-2 Days Before the Procedure/Surgery (as Opposed to Continuing Rivaroxaban (Conditional Recommendation, Very Low Certainty of Evidence)
- The Total Duration of Periprocedural/Perioperative Rivaroxaban Interruption Will Depend on the Bleeding Risk Associated with the Procedure/Surgery
- Low-Moderate Bleeding Risk: 1 day off rivaroxaban prior to procedure/surgery
- High Bleeding Risk: 2 days off rivaroxaban prior to procedure/surgery
- This Management May Be Applied Irrespective of Whether Patients are Receiving Rivaroxaban for Atrial Fibrillation or Venous Thromboembolism
- The Total Duration of Periprocedural/Perioperative Rivaroxaban Interruption Will Depend on the Bleeding Risk Associated with the Procedure/Surgery
- In Patients Receiving DOAC Who Require an Elective Procedure/Surgery, Perioperative Heparin Bridging is Not Recommended (Conditional Recommendation, Very Low Certainty of Evidence)
- The Rapid Offset and Rapid Onset of Action of DOAC’s Obviates the Need for Heparin Bridging with Short-Acting Anticoagulants Such as Unfractionated Heparin or Low Molecular Weight Heparin in a Periprocedural/Perioperative Setting
- In Patients Who Had DOAC Interruption for an Elective Procedure/Surgery, Resume DOAC >24 hrs After the Procedure/Surgery (as Opposed to Resuming Rivaroxaban within 24 hrs (Conditional Recommendation, Very Low Certainty of Evidence)
- The Resumption of Postprocedure/Postoperative DOAC Will Depend on the Bleeding Risk Associated with the Procedure/Surgery
- Low-Moderate Bleeding Risk: resume DOAC at least 24 hrs after procedure/surgery
- High Bleeding Risk: resume DOAC 48-72 hours after procedure/surgery
- DOAC’s Have a Rapid Onset of Action, with a Peak Effect Occurring 1-3 hrs After Intake, Thereby Requiring Cautious Administration After a Procedure/Surgery
- The Resumption of Postprocedure/Postoperative DOAC Will Depend on the Bleeding Risk Associated with the Procedure/Surgery
- In Patients Who Had DOAC Interruption for an Elective Procedure/Surgery, Routine DOAC Coagulation Function Testing is Not Recommended to Guide Periprocedural/Perioperative DOAC Management (Conditional Recommendation, Very Low Certainty of Evidence)
- DOAC Level Testing May Be Considered, on a Case-by-Case Basis, in Non-Elective Periprocedural/Perioperative Clinical Situations
- For Example, in Patients Who Require an Urgent/Emergency Prodedure/Surgery in Whom DOAC Level Testing May Inform the Need for Active DOAC Reversal with Administration of Blood Products or DOAC-Specific Reversal Agents
- DOAC Level Testing May Be Considered, on a Case-by-Case Basis, in Non-Elective Periprocedural/Perioperative Clinical Situations
Reversal of Rivaroxaban Anticoagulation
Activated Charcoal (see Activated Charcoal)
- Clinical Efficacy: indicated to decrease rivaroxaban absorption
Andexanet Alfa (see Andexanet)
- Pharmacology
- Andexanet Alfa is a Modified Recombinant Derivative of Factor Xa Which Acts as a Decoy Receptor
- Andexanet Alfa Has a Higher Affinity for the Factor Xa Inhibitor Drug than Natural Factor Xa
- Consequently Drug Binds to Andexanet Alfa, Rather than Factor Xa Itself
- Andexanet Alfa is a Modified Recombinant Derivative of Factor Xa Which Acts as a Decoy Receptor
- Clinical Efficacy: currently investigational
- ANNEXA-4 Trial of Andexanet Alfa for the Treatment of Major Hemorrhage Associated with Factor Xa Inhibitors (NEJM, 2016) [MEDLINE]
- Andexanet Alfa (Initial Bolus and Subsequent Infusion x 2 hrs) Decreased Factor Xa Activity in Patients with Major Hemorrhage Associated with Factor Xa Inhibitors: effective hemostasis occurred in 79% of patients
- After the Bolus (and Remaining Approximately the Same During the Infusion), Anti-Factor Xa Activity was Decreased 93% from Baseline in Patients on Apixaban
- After the Bolus (and Remaining Approximately the Same During the Infusion), Anti-Factor Xa Activity was Decreased 89% from Baseline in Patients on Rivaroxaban
- Thrombotic Events Occurred in 18% of Patients within 30 Days
- Andexanet Alfa (Initial Bolus and Subsequent Infusion x 2 hrs) Decreased Factor Xa Activity in Patients with Major Hemorrhage Associated with Factor Xa Inhibitors: effective hemostasis occurred in 79% of patients
- ANNEXA-4 Trial of Andexanet Alfa for the Treatment of Major Hemorrhage Associated with Factor Xa Inhibitors (NEJM, 2016) [MEDLINE]
Hemodialysis (see Hemodialysis)
- Clinical Efficacy: unlikely to be effective (due to high degree of rivaroxaban protein binding)
Prothrombin Complex Concentrate-4 Factor (Kcentra, Beriplex, Confidex) (see Prothrombin Complex Concentrate-4 Factor)
- Clinical Efficacy
- Prothrombin Complex Concentrate is Suggested for Rivaroxaban-Associated Hemorrhage (Biomed Res Int, 2014) [MEDLINE]
- Abstract from Rat Study Suggests Efficacy
- Analysis of Bleeding Complications from ROCKET-AF Trial (Eur Heart J, 2014) [MEDLINE]
- In High-Risk Patients with AF and Major Bleeding in ROCKET-AF Trial, the Use of FFP and Prothrombin Complex Concentrate was Less in Patients on Rivaroxaban, as Compared to Those on Coumadin
- However, the Use of PRBC’s and Outcomes After Bleeding were Similar in Both Groups
- Prothrombin Complex Concentrate is Suggested for Rivaroxaban-Associated Hemorrhage (Biomed Res Int, 2014) [MEDLINE]
- Administration: 50 units/kg IV
Recombinant Factor VIIa (see Factor VIIa)
- Clinical Efficacy: pre-clinical trials suggest that this may be effective
Adverse Effects
Hemorrhagic Adverse Effects
Comparative Rates of Hemorrhage Between Coumadin and Novel Oral Anticoagulants
- Systematic Review of Novel Oral Anticoagulants, As Compared to Coumadin (Ann Intern Med, 2012) [MEDLINE]: included chronic non-valvular atrial fibrillation trials (RE-LY Trial (2009): dabigatran, ARISTOTLE Trial (2011): apixaban, ROCKET AF Trial (2011): rivaroxaban) and venous thromboembolism trials (EINSTEIN-DVT (2010): rivaroxaban, RE-COVER (2009): dabigatran, EINSTEIN-PE (2012): rivaroxaban)
- Decreased Risk of Fatal Bleeding, as Compared to Coumadin (RR, 0.60 [CI, 0.46 to 0.77])
- Decreased Risk of Major Bleeding, as Compared to Coumadin (RR, 0.80 [CI, 0.63 to 1.01])
- Increased Risk of Gastrointestinal Bleeding, as Compared to Coumadin (RR, 1.30 [CI, 0.97 to 1.73])
- Increased Risk of Discontinuation Due to Adverse Events, as Compared to Coumadin (RR, 1.23 [CI, 1.05 to 1.44])
- Bleeding Risk for New Oral Anticoagulants May Be Higher in Patients >75 y/o or Those Receiving Coumadin Who Have Good Control
- Systematic Review/Meta-Analysis Comparing Rates of Hemorrhage of Novel Oral Anticoagulants vs Coumadin When Used in the Setting of Renal Insufficiency (Chest, 2016) [MEDLINE]
- CrCl 50-80 mL/min: novel oral anticoagulants had a significantly decreased risk of major bleeding, as compared to coumadin
- CrCl <50 mL/min: novel oral anticoagulants had a non-significantly decreased risk of major bleeding, as compared to coumadin
- Apixaban had the lowest rate of major bleeding in this subgroup
Types of Hemorrhage
- Adrenal Hemorrhage (see Adrenal Insufficiency)
- Diffuse Alveolar Hemorrhage (DAH) (see Diffuse Alveolar Hemorrhage)
- Epistaxis (see Epistaxis)
- Gastrointestinal Hemorrhage (see Gastrointestinal Hemorrhage)
- Hematuria (see Hematuria)
- Intracerebral Hemorrhage (Hemorrhagic Cerebrovascular Accident) (see Intracerebral Hemorrhage)
- Intracranial Epidural Hematoma (see Intracranial Epidural Hematoma)
- Retroperitoneal Hemorrhage (see Retroperitoneal Hemorrhage)
- Spinal Epidural Hematoma (see Spinal Epidural Hematoma)
- Subarachnoid Hemorrhage (SAH) (see Subarachnoid Hemorrhage)
- Subdural Hematoma (SDH) (see Subdural Hematoma)
Other Adverse Effects
- xxx
- xxx
- xxx
References
General
- Prothrombin complex concentrate reverses the effects of high-dose rivaroxaban in rats [abstract]. J Thromb Haemost. 2009;7(suppl 2):183
- Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokin 2009;48(1):1-22 [MEDLINE]
- Bayer Schering Pharma. Xarelto. Summary of Product Characteristics. May 2009. www.xarelto.com/html/downloads/ XareltoSummaryofProductCharacteristics_May2009.pd
- Assessment of laboratory assays to measure rivaroxaban — an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815-825
- Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133-139
- The new oral anticoagulants and the future of haemostasis laboratory testing. Biochem Med (Zagreb). 2012;22:329-341
- Perioperative Management of Antithrombotic Therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest. 2022 Aug 11;S0012-3692(22)01359-9. doi: 10.1016/j.chest.2022.07.025 [MEDLINE]
Indications
Atrial Fibrillation
- Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med. 2012 Dec 4;157(11):796-807 [MEDLINE]
- Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381–2391 [MEDLINE]
- Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e531S-75S. doi: 10.1378/chest.11-2304 [MEDLINE]
- Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962 [MEDLINE]
- Systematic review of cost-effectiveness analyses of novel oral anticoagulants for stroke prevention in atrial fibrillation. Rev Port Cardiol. 2015 Mar;34(3):179-91. doi: 10.1016/j.repc.2014.08.008. Epub 2015 Feb 27 [MEDLINE]
- Cost-effectiveness of novel oral anticoagulants for stroke prevention in non-valvular atrial fibrillation. Curr Cardiol Rep. 2015;17:61 [MEDLINE]
- Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost. 2015 Nov;13(11):2012-20. doi: 10.1111/jth.13139. Epub 2015 Oct 5 [MEDLINE]
Venous Thromboembolism
- RECORD trial: Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br. 2009 May;91(5):636-44. doi: 10.1302/0301-620X.91B5.21691 [MEDLINE]
- Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med. 2012 Dec 4;157(11):796-807 [MEDLINE]
- Systematic review of randomized controlled trials of new anticoagulants for venous thromboembolism prophylaxis in major orthopedic surgeries, compared with enoxaparin. Ann Vasc Surg. 2013 Apr;27(3):355-69. doi: 10.1016/j.avsg.2012.06.010. Epub 2013 Jan 23 [MEDLINE]
- MAGELLAN Trial: Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013 Feb 7;368(6):513-23. doi: 10.1056/NEJMoa1111096 [MEDLINE]
- New oral anticoagulants in the treatment of pulmonary embolism: efficacy, bleeding risk, and monitoring. Thrombosis 2013;2013:973710. doi: 10.1155/2013/973710 [MEDLINE]
- Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320-8. doi: 10.1111/jth.12485 [MEDLINE]
- The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014 Oct 9;124(15):2450-8. doi: 10.1182/blood-2014-07-590323. Epub 2014 Aug 22 [MEDLINE]
- Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost. 2015 Nov;13(11):2012-20. doi: 10.1111/jth.13139. Epub 2015 Oct 5 [MEDLINE]
- The MARINER trial of rivaroxaban after hospital discharge for medical patients at high risk of VTE. Design, rationale, and clinical implications. Thromb Haemost. 2016 Jun 2;115(6):1240-8. doi: 10.1160/TH15-09-0756. Epub 2016 Feb 4 [MEDLINE]
Administration
Administration in Specific Clinical Subsets of Patients
- Major Bleeding and Hemorrhagic Stroke with Direct Oral Anticoagulants in Patients with Renal Failure: Systematic Review and Meta-Analysis of Randomized Trials. Chest. 2016,(): doi:10.1016/j.chest.2015.12.029 [MEDLINE]
- Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016 Jun;14(6):1308-13. doi: 10.1111/jth.13323. Epub 2016 Apr 27 [MEDLINE]
Reversal of Anticoagulation
- Reversal of novel oral anticoagulants in patients with major bleeding. J Thromb Thrombolysis. 2013 Apr;35(3):391-8. doi: 10.1007/s11239-013-0885-0 [MEDLINE]
- Management of the bleeding patient receiving new oral anticoagulants: a role for prothrombin complex concentrates. BioMed Res Int. 2014; Article ID 583794 [MEDLINE]
- Treatment of intracerebral hemorrhage associated with new oral anticoagulant use: the neurologist’s view. Clin Lab Med. 2014;34:587–594 [MEDLINE]
- Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J. 2014 Jul 21;35(28):1873-80. doi: 10.1093/eurheartj/ehu083. Epub 2014 Mar 21 [MEDLINE]
- Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113(5):931–942 [MEDLINE]
- Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–2424 [MEDLINE]
- ANNEXA-4 Trial. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375:1131–1141 [MEDLINE]
Adverse Effects
- xxxxx
