Epidemiology
Demographics
- Incidence
- 3-5 million patients develop severe influenza annually
- 250k-500k patients die of influenza-related causes annually
- Peak Season:
- Prior Exposure: epidemiological and laboratory serology indicate that older people (age >65 y/o) may have pre-existing immunity to the novel H1N1 virus due to a prior exposure to a related virus
Risk Factors for Influenza
- Tobacco (see Tobacco)
- Epidemiology
- Systematic Review of the Effect of Cigarette Smoking on the Risk of Developing Influenza (J Infect, 2019) [MEDLINE]: n = 9 (n = 40,685 participants) with a study quality of 5.4 of 9 (SD: 1.07)
- Current Smokers were >5x More Likely to Develop Laboratory-Confirmed Influenza than Non-Smokers (Pooled Odds Ratio 5.69; 95% CI: 2.79-11.60; 3 Studies)
- For Studies Reporting the Occurrence of an Influenza-Like Illness, Current Smokers were 34% More Likely to Develop Influenza-Like Illness than Non-Smokers (Pooled Odds Ratio 1.34; 95% CI: 1.13-1.59; 6 Studies)
- Systematic Review of the Effect of Cigarette Smoking on the Risk of Developing Influenza (J Infect, 2019) [MEDLINE]: n = 9 (n = 40,685 participants) with a study quality of 5.4 of 9 (SD: 1.07)
- Epidemiology
Viral Epidemiology
- Over 99% of Influenza Infections are Due to Influenza A
- Influenza A-H3N2: most common circulating influenza A serotype in the US in the past decades
- Leads to more severe disease and higher rates of hosptilization/death than influenza B
- Generally associated with higher viral loads
- Influenza A-H1N1: lowest severity index (lowest morbidity/mortality)
- Generally associated with lower viral loads
- Influenza A-H3N2: most common circulating influenza A serotype in the US in the past decades
H7N9 Virus
Epidemiology of Avian Influenza H7N9 Infection in China (NEJM, 2014) [MEDLINE]
- Median Age: 61 y/o
- Sex: 71% of cases were male
- Exposure: 82% of cases had exposure to chickens
- Transmission: in four family clusters, human-to-human transmission could not be ruled out
- Excluding these family clusters, only 1% of close contacts of case patients developed respiratory symptoms
- Clinical Severity
- 90% of cases had pneumonia or respiratory failure
- 63% of cases were admitted to an ICU
- Mortality Rate: 34% (with median duration of illness: 21 days)
Factors Which Predict High Risk for Influenza Complications (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
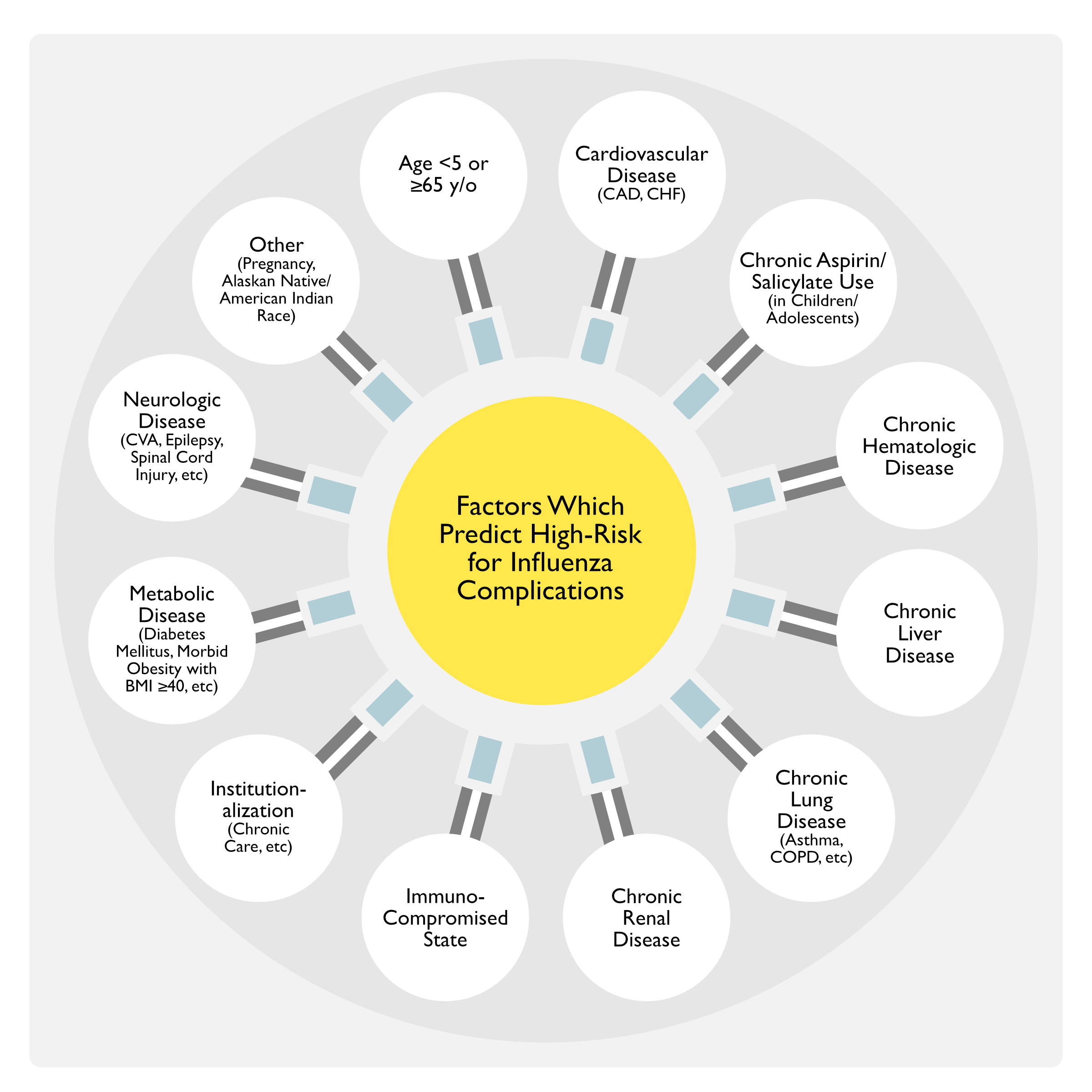
Age
- Adults ≥65 y/o
- Children <5 y/o
- Highest Risk in Children <2 y/o
Cardiovascular Disease (Except Hypertension Alone)
- Coronary Artery Disease (CAD) (see Coronary Artery Disease)
- Epidemiology
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- There were 2,435 Severe Influenza-Associated Hospitalizations
- Asthma: Incidence Rate Ratio Range: 4.07–5.02 Across Age Strata
- Chronic Obstructive Pulmonary Disease: Incidence Rate Ratio Range: 5.89–8.78 Across Age Strata
- Congestive Heart Failure: Incidence Rate Ratio Range: 4.84–13.4 Across Age Strata
- Coronary Artery Disease: Incidence Rate Ratio Range: 2.77–10.7 Across Age Strata
- Diabetes Mellitus: Incidence Rate Ratio Range: 2.17–2.30 in Age 18-64 y/o Group (Incidence Rate Ratio Range: 0.903-1.64 in Age 65-80 y/o Group)
- End-Stage Renal Disease: Incidence Rate Ratio Range: 3.30–9.02 Across Age Strata
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- Epidemiology
- Congestive Heart Failure (CHF) (see Congestive Heart Failure)
- Epidemiology
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- There were 2,435 Severe Influenza-Associated Hospitalizations
- Asthma: Incidence Rate Ratio Range: 4.07–5.02 Across Age Strata
- Chronic Obstructive Pulmonary Disease: Incidence Rate Ratio Range: 5.89–8.78 Across Age Strata
- Congestive Heart Failure: Incidence Rate Ratio Range: 4.84–13.4 Across Age Strata
- Coronary Artery Disease: Incidence Rate Ratio Range: 2.77–10.7 Across Age Strata
- Diabetes Mellitus: Incidence Rate Ratio Range: 2.17–2.30 in Age 18-64 y/o Group (Incidence Rate Ratio Range: 0.903-1.64 in Age 65-80 y/o Group)
- End-Stage Renal Disease: Incidence Rate Ratio Range: 3.30–9.02 Across Age Strata
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- Epidemiology
Chronic Aspirin/Salicylate Use in Children/Adolescents (see Acetylsalicylic Acid)
- Chronic Aspirin Therapy with Superimposed Influenza Increases the Risk of Reye’s Syndrome
- Chronic Aspirin Therapy May Be Used in Conditions Such as Kawasaki Disease, Rheumatoid Arthritis, etc (see Kawasaki Disease and Rheumatoid Arthritis)
Chronic Hematologic Disease
- Sickle Cell Disease (see Sickle Cell Disease)
- Other Hemoglobinopathies
Chronic Liver Disease
- Cirrhosis (see Cirrhosis)
- Epidemiology
- XXXXX
- Epidemiology
Chronic Lung Disease
- Asthma (see Asthma)
- Epidemiology
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- There were 2,435 Severe Influenza-Associated Hospitalizations
- Asthma: Incidence Rate Ratio Range: 4.07–5.02 Across Age Strata
- Chronic Obstructive Pulmonary Disease: Incidence Rate Ratio Range: 5.89–8.78 Across Age Strata
- Congestive Heart Failure: Incidence Rate Ratio Range: 4.84–13.4 Across Age Strata
- Coronary Artery Disease: Incidence Rate Ratio Range: 2.77–10.7 Across Age Strata
- Diabetes Mellitus: Incidence Rate Ratio Range: 2.17–2.30 in Age 18-64 y/o Group (Incidence Rate Ratio Range: 0.903-1.64 in Age 65-80 y/o Group)
- End-Stage Renal Disease: Incidence Rate Ratio Range: 3.30–9.02 Across Age Strata
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- Epidemiology
- Bronchiectasis (if Clinically Significant) (see Bronchiectasis)
- Chronic Obstructive Pulmonary Disease (COPD) (see Chronic Obstructive Pulmonary Disease)
- Epidemiology
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- There were 2,435 Severe Influenza-Associated Hospitalizations
- Asthma: Incidence Rate Ratio Range: 4.07–5.02 Across Age Strata
- Chronic Obstructive Pulmonary Disease: Incidence Rate Ratio Range: 5.89–8.78 Across Age Strata
- Congestive Heart Failure: Incidence Rate Ratio Range: 4.84–13.4 Across Age Strata
- Coronary Artery Disease: Incidence Rate Ratio Range: 2.77–10.7 Across Age Strata
- Diabetes Mellitus: Incidence Rate Ratio Range: 2.17–2.30 in Age 18-64 y/o Group (Incidence Rate Ratio Range: 0.903-1.64 in Age 65-80 y/o Group)
- End-Stage Renal Disease: Incidence Rate Ratio Range: 3.30–9.02 Across Age Strata
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- Epidemiology
- Cystic Fibrosis (CF) (see Cystic Fibrosis)
- Interstitial Lung Disease (ILD) (see Interstitial Lung Disease)
Chronic Renal Disease
- Chronic Kidney Disease (CKD) (see Chronic Kidney Disease)
- Epidemiology
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- There were 2,435 Severe Influenza-Associated Hospitalizations
- Asthma: Incidence Rate Ratio Range: 4.07–5.02 Across Age Strata
- Chronic Obstructive Pulmonary Disease: Incidence Rate Ratio Range: 5.89–8.78 Across Age Strata
- Congestive Heart Failure: Incidence Rate Ratio Range: 4.84–13.4 Across Age Strata
- Coronary Artery Disease: Incidence Rate Ratio Range: 2.77–10.7 Across Age Strata
- Diabetes Mellitus: Incidence Rate Ratio Range: 2.17–2.30 in Age 18-64 y/o Group (Incidence Rate Ratio Range: 0.903-1.64 in Age 65-80 y/o Group)
- End-Stage Renal Disease: Incidence Rate Ratio Range: 3.30–9.02 Across Age Strata
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- Epidemiology
Immunosuppression
- Human Immunodeficiency Virus (HIV) (see Human Immunodeficiency Virus)
- Medication-Induced
- Corticosteroids (see Corticosteroids)
Institutionalization
- Chronic Care Facility Resident
- Nursing Home Resident
Metabolic Disease
- Diabetes Mellitus (DM) (see Diabetes Mellitus)
- Epidemiology
- Diabetes Mellitus Increased the Risk of Influenza-Associated Hospital Admission and Death (BMJ, 2013) [MEDLINE]
- New Zealand Study of Risk Factors for Severe Influenza (J Infect Dis, 2020) [MEDLINE]: n = 891, 276 adults (age 18–80 y/o)
- There were 2,435 Severe Influenza-Associated Hospitalizations
- Asthma: Incidence Rate Ratio Range: 4.07–5.02 Across Age Strata
- Chronic Obstructive Pulmonary Disease: Incidence Rate Ratio Range: 5.89–8.78 Across Age Strata
- Congestive Heart Failure: Incidence Rate Ratio Range: 4.84–13.4 Across Age Strata
- Coronary Artery Disease: Incidence Rate Ratio Range: 2.77–10.7 Across Age Strata
- Diabetes Mellitus: Incidence Rate Ratio Range: 2.17–2.30 in Age 18-64 y/o Group (Incidence Rate Ratio Range: 0.903-1.64 in Age 65-80 y/o Group)
- End-Stage Renal Disease: Incidence Rate Ratio Range: 3.30–9.02 Across Age Strata
- Epidemiology
Morbid Obesity
- Morbid Obesity (BMI ≥40) (see Obesity)
- Epidemiology
- XXXXX
- Epidemiology
Neurologic/Neurodevelopmental Disease
- Cerebral Palsy (see Cerebral Palsy)
- Cerebrovascular Accident (see Ischemic Cerebrovascular Accident)
- Chronic Spinal Cord Injury (see Chronic Spinal Cord Injury)
- Developmental Delay
- Epilepsy (see Seizures)
- Mental Retardation
- Muscular Dystrophy
Pregnancy (see Pregnancy)
- Pregnancy/Post-Partum (Within 2 Weeks of Delivery)
Race
- Alaskan Native Race
- American Indian Race
Virology and Physiology
Influenza Virus is a Member of Orthomyxovirus Family (Orthomyxoviridae) (see Orthomyxoviruses)
- Orthomyxoviruses are RNA Viruses
Transmission of Influenza Virus
- Transmission of Influenza Virus Occurs Predominantly Via Large (>5 Micron) Particle Droplets
- Large Amounts of Influenza Virus are Present in Respiratory Secretions of Infected Influenza Patients
- Large Particles Travel a Limited (Short) Distance: approximately 6 feet
- While Small Particles (Which Can Become Aerosolized and Remain Suspended in the Air) Contain Influenza Virus, Their Contribution to Transmission of Influenza is Unclear
- While Contact with Surfaces Which Have Been Contaminated by Respiratory Droplets is a Potential Source of Transmission, These are Not an Established Mode of Transmission
- Trans-Ocular Transmission of Influenza Virus Has Also Been Documented in Studies (J Infect Dis, 2011) [MEDLINE]
Incubation
- Incubation Period: 1-4 days
- Average Incubation Period: 2 days
Impact of Influenza Virus Infection on the Lung (Am J Respir Cell Mol Biol, 2019) [MEDLINE]
- Influenza Virus Damages the Respiratory Epithelium (J Virol, 2018) [MEDLINE]
- Influenza Virus Damages Alveolar Barrier by Disrupting Epithelial Tight Junctions (Eur Respir J, 2016) [MEDLINE]
Viral Shedding
- Viral Shedding in Otherwise Healthy Adults Infected with Influenza Virus Can Be Detected 24-48 hrs Before the Onset of Illness
- However, this Viral Shedding is at Much Lower Titers than Those Observed During the Symptomatic Period
- Viral Shedding Increases 12-24 hrs Following Exposure, Peaks at 2 Days, and Then Rapidly Declines (Am J Epidemiol, 2008) [MEDLINE]
- Average Duration of Viral Shedding: 4.8 days (range: 4.3-5.3 days)
- Shedding Generally Ceases After 6-7 Days (Although May Persist Up to 10 Days in Some Cases)
- Longer Periods of Viral Shedding May Occur in Children, Older Adults, Patients with Chronic Diseases, and Immunocompromised Patients
Diagnosis
Virologic Testing for Any Patient Presenting with an Acute Respiratory Illness
In Any Patient Presenting with an Acute Respiratory Illness, an Oropharyngeal Swab with Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) Testing for SARS-CoV-2 is Required for the Purpose of Infection Control (see Severe Acute Respiratory Syndrome Coronavirus-2)
Recommendations (Infectious Diseases Society of America, IDSA/American Thoracic Society, ATS 2007 Consensus Guidelines for the Management of CAP) (Clin Infect Dis, 2007) [MEDLINE]
- Patients with Illness Compatible with Influenza and Known Exposure to Poultry in Areas with Previous H5N1 Infection Should be Tested for H5N1 (Moderate Recommendation, Level III Evidence)
- In Patients with Suspected H5N1 Infection, Droplet Precautions and Careful Routine Infection Control Measures Should Be Used Until H5N1 Infection is Ruled Out (Moderate Recommendation, Level III Evidence)
Recommendations (Infectious Diseases Society of America Clinical Practice Guidelines for Seasonal Influenza, 2009) (Clin Infect Dis, 2009) [MEDLINE]
- Patients Who Should Be Tested for Influenza During Influenza Season (if Testing Will Influence Clinical Management)
- Outpatient Immunocompetent Patients of Any Age at High Risk of Developing Influenza Complications (Hospitalization, Death) Presenting with Acute Febrile Respiratory Symptoms, Within 5 Days After Onset of Illness
- Five Days Corresponds to Period of Time When Influenza Virus is Usually Being Shed in Immunocompetent Patients
- Outpatient Immunocompromised Patients of Any Age Presenting with Febrile Respiratory Symptoms, Irrespective of Time Since Onset of Illness
- Immunocompromised Patients Can Shed Influenza Virus for Weeks-Months
- Hospitalized Patients of Any Age (Immunocompetent or Immunocompromised) with Fever and Respiratory Symptoms (Including Those with a Diagnosis of Community-Acquired Pneumonia), Irrespective of Time Since Onset of Illness
- Elderly Patients and Infants Presenting with Suspected Sepsis or Fever of Unknown Origin, Irrespective of Time Since Onset of Illness
- Children with Fever and Respiratory Symptoms Presenting for Medical Evaluation, Irrespective of Time Since Onset of Illness
- Patients of Any Age Who Develop Fever and Respiratory Symptoms After Hospital Admission, Irrespective of Time Since Onset of Illness
- Immunocompetent Persons with Acute Febrile Respiratory Symptoms Who are Not at High Risk of Developing Complications Secondary to Influenza Infection May Be Tested for Purposes of Obtaining Local Surveillance Data
- Outpatient Immunocompetent Patients of Any Age at High Risk of Developing Influenza Complications (Hospitalization, Death) Presenting with Acute Febrile Respiratory Symptoms, Within 5 Days After Onset of Illness
- Patients Who Should Be Tested for Influenza at Any Time of the Year
- Health Care Personnel, Residents or Visitors in an Institution Experiencing an Influenza Outbreak Who Present with Febrile Respiratory Symptoms, Within 5 Days After Onset of Illness
- Persons Who are Epidemiologically Linked to an Influenza Outbreak (Household and Close Contacts of Patients with Suspected Influenza, Returning Travelers from Countries Where Influenza Viruses May Be Circulating, Participants in International Mass Gatherings, and Cruise Ship Passengers), Who Present Within 5 Days After Onset of Illness
Recommendations for Diagnostic Testing for Influenza (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
Which Patients Should Be Tested for Influenza
- Outpatients (including the Emergency Department)
- During Influenza Activity (Defined as the Circulation of Seasonal Influenza A/B Viruses in the Local Community)
- Clinicians Should Test for Influenza in High-Risk Patients, Including Immunocompromised Patients Who Present with Influenza-Like Illness, Pneumonia, or Nonspecific Respiratory Illness (i.e. Cough without Fever) if the Testing Result Will Influence Clinical Management (A–III)
- Clinicians Should Test for Influenza in Patients Who Present with Acute Onset of Respiratory Symptoms with/without Fever, and Either Exacerbation of Chronic Medical Conditions (Asthma, Chronic Obstructive Pulmonary Disease, Heart Failure) or Known Complications of Influenza (Pneumonia) if the Testing Result Will Influence Clinical Management (A-III)
- Clinicians Can Consider Influenza Testing for Patients Not at High Risk for Influenza Complications Who Present with Influenza-Like Illness, Pneumonia, or Nonspecific Respiratory Illness (i.e. Cough without Fever) and Who are Likely to Be Discharged Home if the Results Might Influence Antiviral Treatment Decisions or Reduce the Use of Unnecessary Antibiotics, Further Diagnostic Testing, and Time in the Emergency Department, or if the Results Might Influence Antiviral Treatment or Chemoprophylaxis Decisions for High-Risk Household Contacts (C-III)
- During Low Influenza Activity without Any Link to an Influenza Outbreak
- Clinicians Can Consider Influenza Testing in Patients with Acute Onset of Respiratory Symptoms with/without Fever (Especially for Immunocompromised and High-Risk Patients) (B-III)
- During Influenza Activity (Defined as the Circulation of Seasonal Influenza A/B Viruses in the Local Community)
- Inpatients
- During Influenza Activity (Defined as the Circulation of Seasonal Influenza A/B Viruses in the Local Community)
- Clinicians Should Test for Influenza on Admission in All Patients Requiring Hospitalization with Acute Respiratory illness (Including pneumonia) with/without Fever (A-II)
- Clinicians Should Test for Influenza on Admission in All Patients with Acute Worsening of Chronic Cardiopulmonary Disease (Asthma, Chronic Obstructive Pulmonary Disease, Coronary Artery Disease, or Heart Failure), as Influenza Can Be Associated with Exacerbation of Underlying Conditions (A-III)
- Clinicians Should Test for Influenza on Admission in All Patients Who are Immunocompromised or at High Risk of Complications and Present with Acute Onset of Respiratory Symptoms with/without Fever, as the Manifestations of Influenza in Such Patients are Frequently Less Characteristic than in Immunocompetent Individuals (A-III)
- Clinicians Should Test for Influenza in All Patients Who, While Hospitalized, Develop Acute Onset of Respiratory Symptoms, with/without Fever, or Respiratory Distress, without a Clear Alternative Diagnosis (A-III)
- During Low Influenza Activity without Any Link to an Influenza Outbreak
- Clinicians Should Test for Influenza on Admission in All Patients Requiring Hospitalization with Acute Respiratory Illness, with/without Fever, Who Have an Epidemiological Link to a Patient Diagnosed with Influenza, an Influenza Outbreak or Outbreak of Acute Febrile Respiratory Illness of Uncertain Etiology, or Who Recently Traveled from an Area with Known Influenza Activity (A-II)
- Clinicians Can Consider Testing for Influenza in Patients with Acute, Febrile Respiratory Tract illness, Especially Children and Adults Who are Immunocompromised or at High Risk of Influenza Complications, or if the Results Might Influence Antiviral Treatment or Chemoprophylaxis Decisions for High-Risk Household Contacts (B-III)
- During Influenza Activity (Defined as the Circulation of Seasonal Influenza A/B Viruses in the Local Community)
What Specimen(s) Should Be Collected When Testing Patients for Influenza
- Clinicians Should Collect upper respiratory tract specimens from outpatients for influenza testing as soon after illness onset as possible, preferably within 4 days of symptom onset (A-II)
- Nasopharyngeal Specimens Should Be Collected Over Other Upper Respiratory Tract Specimens to Increase Detection of Influenza Viruses (A-II)
- If nasopharyngeal specimens are not available, nasal and throat swab specimens should be collected and combined together for influenza Testing over single specimens from either site (particularly over throat swabs) to increase detection of Influenza Viruses (A-II)
- Mid-turbinate nasal swab specimens should be collected over throat swab specimens to increase detection of influ- enza viruses (A-II)
- Flocked swab specimens should be collected over non-flocked swab specimens to improve detection of Influenza viruses (A-II)
- Clinicians Should Collect Nasopharyngeal (optimally, as for outpatients), mid-turbinate nasal, or combined nasal–throat specimens from hospitalized patients without severe lower respiratory tract disease for Influenza Testing as Soon as Possible (A-II)
- Clinicians Should Collect endotracheal aspirate or bronchoalveolar lavage fluid specimens from hospitalized patients with respiratory failure receiving mechanical ventilation, including patients with negative Influenza Testing results on upper respiratory tract specimens, for Influenza testing as soon as possible (A-II)
- Clinicians Should Not collect or routinely test specimens for influenza from nonrespiratory sites such as blood, plasma, serum, cerebrospinal fluid, urine, and stool (A-III)
- Clinicians Should Not Collect Serum Specimens (Including Single or Paired Sera) for Serological Diagnosis of Seasonal Influenza Virus Infection for Clinical Management Purposes (A-III)
Which Tests Should Be Used to Diagnose Influenza
- Outpatient
- Clinicians Should Use Rapid Molecular Assays (Nucleic Acid Amplification Tests) Over Rapid Influenza Diagnostic Tests (RIDT’s) in Outpatients to Improve Detection of Influenza Virus Infection (A-II)
- Inpatient
- Clinicians Should Use Reverse-Transcription Polymerase Chain Reaction (RT-PCR) or Other Molecular Assays Over Other Influenza Tests in Hospitalized Patients to Improve Detection of Influenza Virus Infection (A-II)
- Clinicians Should Use Multiplex RT-PCR Assays Targeting a Panel of Respiratory Pathogens, Including influenza Viruses, in Hospitalized Immunocompromised Patients (A-III)
- Clinicians Can Consider Using Multiplex RT-PCR Assays Targeting a Panel of Respiratory Pathogens, Including Influenza Viruses, in Hospitalized Patients Who are Not Immunocompromised if it Might Influence Care (Such as Aid in Cohorting Decisions, Reduce Testing, or Decrease Antibiotic Use) (B-III)
- Clinicians Should Not Use Immunofluorescence Assays for Influenza Virus Antigen Detection in Hospitalized Patients Except When More Sensitive Molecular Assays are Not Available (A-II), and Follow-Up Testing with RT-PCR or Other Molecular Assays Should Be Performed to Confirm Negative Immunofluorescence Test Results (A-III)
- Clinicians Should Not Use RIDT’s in Hospitalized Patients Except When More Sensitive Molecular Assays are Not Available (A-II), and Follow-Up Testing with RT-PCR or Other Molecular Assays Should Be Performed to Confirm Negative RIDT Results (A-II)
- Clinicians Should Not Use Viral Culture for Initial or Primary Diagnosis of Influenza Because Results Will Not Be Available in a Timely Manner to Inform Clinical Management (A-III), But Viral Culture Can Be Considered to Confirm Negative Test Results from RIDT’s and Immunofluorescence Assays, Such as During an Institutional Outbreak, and to Provide Isolates for Further Characterization (C-II)
- Clinicians Should Not Use Serologic Testing for Diagnosis of Influenza Because Results from a Single Serum Specimen Cannot Be Reliably Interpreted, and Collection of Paired (Acute/Convalescent) Sera 2–3 wks Apart are Needed for Serological Testing (A-III)
Immunochromatographic Assay (Rapid Influenza Diagnostic Tests = RIDT)
- Clinical Utility
- RIDT’s are Useful to “Rule In” Influenza (Due to Higher Specificity), But Not Useful to “Rule Out” Influenza (Due to Highly Variable Sensitivity): due to lower sensitivity, a negative RIDT result should be followed by RT-PCR testing (Clin Infect Dis, 2009) [MEDLINE]
- Cost: inexpensive ($15-20 per assay)
- Sensitivity
- Sensitivity of 70-90% in Children: perform better in children due to higher viral loads and longer period of viral shedding in children
- Sensitivity of <40-60% in Adults
- Performance of RIDT’s Depends Heavily on Patient Age, Duration of Illness, Sample Type, and Perhaps Viral Type
- RIDT’s Have Higher Sensitivity in Detecting Influenza A than Influenza B
- Specificity: typically >90%
- Technique
- Specimen Quality: probably, nasopharygneal > nasal > throat (although some studies do not cite a difference in quality between these specimen sites)
- Common Assays
- Binax Tests: BinaxNOW Flu A and Flu B and Binax-NOW Influenza A + B (Inverness Medical Innovations, Portland, Maine)
- Directigen Tests: Directigen Flu A and Directigen Flu A + B (Becton, Dickinson and Company, Franklin Lakes, New Jersey)
- QuickVue Tests: QuickVue Influenza and QuickVue Influenza A + B (Quidel Corporation, San Diego, California)
- Laboratory Turnaround Time: 15-30 min (may be used at the point of care)
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
- Clinical Utility
- RT-PCR is the Gold Standard Test
- RT-PCR is the Most Sensitive and Specific Test for Influenza (Clin Infect Dis, 2009) [MEDLINE]: it has a 2-13% higher detection rate than viral culture
- RT-PCR Has Very High Specificity for Influenza
- RT-PCR is Useful for Differentiating Between Influenza Types/Subtypes
- RT-PCR is the Preferred Test for Patients with a History of Exposure to Animals with Possible Influenza
- Influenza A-H5N1 in Poultry in Eurasia or Africa
- Swine Influenza in Any Part of the World (Including North America)
- RT-PCR is the Least Widely Available Test: due to need for specialized expertise
- Cost: RT-PCR is the most expensive diagnostic test
- Technique
- Specimen: upper or lower respiratory tract specimen
- Endotracheal Aspirate or Bronchoalveolar Lavage (BAL) Specimens Have Higher Yields in Patients with Lower Respiratory Tract Illness
- Methods
- Conventional Gel-Based PCR
- Real-Time RT-PCR
- Multiplex PCR
- Laboratory Turnaround Time: 2 hrs (although if samples are run in batches in clinical labs, delayed testing may occur)
- Specimen: upper or lower respiratory tract specimen
Immunofluorescence
- Clinical Utility
- Slightly Lower Sensitivity and Specificity than Viral Isolation in Cell Culture
- Technique
- Specimen: must contain respiratory epithelial cells to achieve reasonable quality of assay result
- Methods: performance of assay depends on heavily on laboratory expertise and quality of the specimen collected
- Direct Fluorescent Antibody Staining: detects and distinguishes between influenza A and B and between A/B and other respiratory viruses
- Indirect Fluorescent Antibody Staining: detects and distinguishes between influenza A and B and between A/B and other respiratory viruses
- Laboratory Turnaround Time: 2-4 hrs
Neuraminidase Detection Assay
- Clinical Utility
- Neuraminidase Detection Assay Detects But Does Not Distinguish Between Influenza A and B
- Technique
- Laboratory Turnaround Time: 20-30 min
Influenza Viral Culture
- Clinical Utility
- Historical Viral Culture was the Gold Standard Diagnostic Test: but has recently been replaced by RT-PCR
- Sensitivity: moderately high
- Specificity: highest of all influenza assays (this makes viral culture important for confirming screening test results and for public health surveillance, but not useful for timely clinical management)
- Technique
- Method
- Shell Vial Culture: 48-72 hr laboratory turnaround time
- Isolation in Cell Culture: 3-10 day laboratory turnaround tim
- Method
Serologic Tests
- Clinical Utility
- Recommended Only for Retrospective Diagnosis, Surveillance, or Research Purposes
- Technique
- Methods
- Hemagglutinin Inhibition
- ELISA
- Complement Fixation
- Neutralization
Clinical Differentiation of Upper Respiratory Tract Infection vs Lower Respiratory Tract Infection
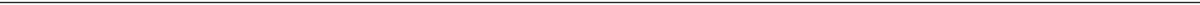
Clinical Manifestations
General Comments
- Incubation Period: 1-4 days
- Average Incubation Period: 2 days
- Onset of Illness
- Usually Abrupt Onset of Fever, Headache, Myalgias, and Malaise
- Associated with Dry Cough, Pharyngitis, and Nasal Discharge
- Usually Abrupt Onset of Fever, Headache, Myalgias, and Malaise
Patient Groups with the Highest Mortality and Hospitalization Rates
- Groups with Highest Risk of Mortality and Serious Morbidity (Hospitalization) (Clin Infect Dis, 2009) [MEDLINE]
- Severely Immunocompromised Patients (Hematopoietic Stem Cell Transplant, etc)
- Very Elderly (Age >85 y/o) Nursing Home Residents
- Infants <24 Months Old: this group has higher hospitalization rate, but lower case-fatality rate than the first two groups
Constitutional Manifestations
Fever (see Fever)
- Clinical
- Fever is Typically 37.8-40.0°C (100-104°F)
- May Be as High as 106°F in Some Cases
- Fever is Frequently Higher in Children than in Adults
- Retrospective, Pooled Analysis of Clinical Symptoms from Zanamivir Trials (Arch Intern Med, 2000) [MEDLINE]: n = 3,744 patients enrolled with baseline influenza-like symptoms
- Approximately 66% of Trial Participants were Found to Have Influenza
- Patients with Influenza were More Likely to Have Cough (93% vs 80%), Fever (68% vs 40%), Cough and Fever Together (64% vs 33%), and/or Nasal Congestion (91% vs 81%) Than Those without Influenza
- Best Multivariate Predictors of Influenza Infection (vs Non-Influenza Infection) was Cough and Fever (Positive Predictive Value = 79% (p< 0.001)
- Positive Predictive Value Rose with the Increase in the Temperature at the Time of Recruitment
- Fever is Typically 37.8-40.0°C (100-104°F)
Cardiovascular Manifestations
- Acute Pericarditis (see Acute Pericarditis)
- Epidemiology
- Uncommon
- May Occur in Infants/Preschool Children/School-Aged Children/Adults
- Epidemiology
- Electrocardiographic (EKG) Changes (see Electrocardiogram)
- Epidemiology
- May Be Due to Underlying Cardiac Disease or Due to Influenza Infection Itself (Observed in Some Cases of Influenza in Young Previously Healthy Patients) (Clin Infect Dis, 2005) [MEDLINE]
- Clinical
- Absence of elevated troponin or echocardiographic abnormalities (in cases due to influenza virus infection itself) (Clin Infect Dis, 2005) [MEDLINE]
- Epidemiology
- Exacerbation of Underlying Cardiovascular Disease
- Clinical
- Congestive Heart Failure (CHF) Exacerbation (see Congestive Heart Failure)
- Clinical
- Myocardial Infarction (see Coronary Artery Disease)
- Epidemiology
- Increased Risk of Myocardial Infarction Has Been Identified in Epidemiological Studies of Seasonal Influenza Outbreaks
- Physiology
- Influenza May Act as a Trigger for Acute Coronary Syndromes
- Epidemiology
- Myocarditis (see Myocarditis)
- Epidemiology
- Uncommon
- May Occur in Infants/Preschool Children/School-Aged Children/Adults
- Physiology: likely not directly due to the influenza virus itself (due to lack of viral antigens in myocardial tissue)
- Epidemiology
Gastrointestinal Manifestations
- Nausea/Vomiting/Diarrhea (see Nausea and Vomiting and Diarrhea)
- Epidemiology
- Uncommon in Adults, But Can Occur in 10-20% of Childhood Cases
- Epidemiology
Infectious Manifestations
- Neisseria Meningitidis Infection (see Neisseria Meningitidis)
- Epidemiology
- XXXX
- Epidemiology
- Staphylococcal Toxic Shock Syndrome (see Staphylococcal Toxic Shock Syndrome)
- Epidemiology
- Has Been Reported with Influenza (Most Commonly Influenza B) and Staphylococus Aureus Co-Infection (Clin Infect Dis, 1993) [MEDLINE]
- Epidemiology
Neurologic Manifestations
- Aseptic Meningitis (see Meningitis)
- Epidemiology
- Has Been Reported
- Epidemiology
- Encephalitis (see Encephalitis)
- Epidemiology
- May Occur in Children/Adults
- Epidemiology
- Encephalopathy (see Delirium)
- Epidemiology
- Has Been Reported
- Epidemiology
- Febrile Seizures (see Seizures)
- Epidemiology
- Predominantly in Infants/Preschool Children
- Reported in 6-20% of Children Hospitalized with Influenza
- Epidemiology
- Guillain-Barre Syndrome (GBS) (see Guillain-Barre Syndrome)
- Epidemiology
- Has Been Reported
- Epidemiology
- Reye Syndrome (see Reye Syndrome)
- Epidemiology
- Predominantly in Infants/Children
- Clinical
- Encephalopathy (see Encephalopathy)
- Elevated Liver Function Tests (see Elevated Liver Function Tests)
- Epidemiology
- Transverse Myelitis (see Transverse Myelitis)
- Epidemiology
- Has Been Reported
- Epidemiology
Otolaryngologic Manifestations
- Acute Rhinosinusitis (see Acute Rhinosinusitis)
- Epidemiology
- May Occur in Children/Adults
- Epidemiology
- Acute Otitis Media (see Acute Otitis Media)
- Epidemiology
- Predominantly in Infants/Children
- Epidemiology
- Parotitis (see Parotitis)
- Epidemiology
- May Occur in Infants/Children/Adults
- Epidemiology
- Rhinorrhea/Nasal Discharge (se Rhinorrhea)
- Epidemiology
- Common
- Epidemiology
- Pharyngitis (see Pharyngitis)
- Epidemiology
- Common
- Epidemiology
Pulmonary Manifestations
Acute Respiratory Distress Syndrome (ARDS) (see Acute Respiratory Distress Syndrome)
- Epidemiology
Bronchiolitis (see Bronchiolitis)
- Epidemiology
- Predominantly in Infants/Preschool Children
Croup (see Croup)
- Epidemiology
- Predominantly in Infants/Preschool Children
Dry Cough/Bronchitis (see Cough)
- Epidemiology
- Dry Cough is Common
- Predominately in School-Aged Childen/Adults
- Physiology
- Clinical
- Normal Lung Exam (In Uncomplicated Influenza) (Am Rev Respir Dis, 1976) [MEDLINE]
- However, in These Patients, Increased A-a Gradient Hypoxemia and Mild Ventilatory Defects May Occur
- Retrospective, Pooled Analysis of Clinical Symptoms from Zanamivir Trials (Arch Intern Med, 2000) [MEDLINE]: n = 3,744 patients enrolled with baseline influenza-like symptoms
- Approximately 66% of Trial Participants were Found to Have Influenza
- Patients with Influenza were More Likely to Have Cough (93% vs 80%), Fever (68% vs 40%), Cough and Fever Together (64% vs 33%), and/or Nasal Congestion (91% vs 81%) Than Those without Influenza
- Best Multivariate Predictors of Influenza Infection (vs Non-Influenza Infection) was the Presence of Both Cough and Fever (Positive Predictive Value = 79%; p< 0.001)
- Positive Predictive Value Rose with the Increase in the Temperature at the Time of Recruitment
- Normal Lung Exam (In Uncomplicated Influenza) (Am Rev Respir Dis, 1976) [MEDLINE]
Exacerbation of Underlying Lung Disease
- Clinical
- Asthma Exacerbation (see Asthma)
- Chronic Obstructive Pulmonary Disease (COPD) Exacerbation (see Chronic Obstructive Pulmonary Disease)
Invasive Pulmonary Aspergillosis (see Invasive Pulmonary Aspergillosis)
- Epidemiology
- Diagnosis
- Optimal Diagnostic Testing Has Not Been Established
- Prognosis
- May Be Associated with an Increased Influenza Mortality Rate (Clin Infect Dis, 2020) [MEDLINE]
- Treatment
- Optimal Treatment Has Not Been Established
Necrotizing Tracheobronchitis (see Acute Bronchitis)
- Epidemiology
- Cases Have Been Reported in Patients in the Setting of Influenza and Staphylococcus Aureus Coinfection (see Staphylococcus Aureus) (Int J Legal Med, 2005) [MEDLINE] (Tuberc Respir Dis (Seoul), 2015) [MEDLINE] (J Bronchology Interv Pulmonol, 2017) [MEDLINE] (Infection, 2018) [MEDLINE]
- Diagnosis
- Bronchoscopy (see Bronchoscopy)
- Sloughing Mucosa
- Bronchoscopy (see Bronchoscopy)
Pleural Effusion (see Pleural Effusion-Exudate)
- Epidemiology
- May Occur in Some Cases
Primary Influenza Pneumonia (see Pneumonia and Interstitial Lung Disease)
- Epidemiology
- Primary Influenza Pneumonia is the Least Common Pneumonic Complication of Influenza
- Risk Factors
- Chronic Lung Disease (COPD, etc) (see Chronic Obstructive Pulmonary Disease)
- Increased Left Atrial Pressure (CHF, etc)
- However, Primary Influenza Pneumonia May Occur in Patients without Other Underlying Disease
- Diagnosis
- Chest X-Ray/Chest CT (see Chest X-Ray and Chest Computed Tomography)
- Bilateral Reticular/Reticulonodular Infiltrates with Lower Lobe Predominance: most common pattern
- Consolidation: may be present with/without reticulonodular infiltrates
- High-Resolution Chest CT (HRCT) (see High-Resolution Chest Computed Tomography)
- Multifocal Peribronchovascular or Subpleural Consolidations and/or Ground Glass Opacities
- Chest X-Ray/Chest CT (see Chest X-Ray and Chest Computed Tomography)
- Clinical: clinical manifestations may be severe (Eur J Clin Microbiol Infect Dis, 1998) [MEDLINE]
Reactive Airway Disease (see Reactive Airway Disease)
- Epidemiology
- May Occur in Children/Adults
Secondary Bacterial Pneumonia (Superinfection)
- Epidemiology
- Synergistic Lethality of Influenza and Bacterial Coinfection Has Been Observed in Animal Models Since Shortly After Influenza Viruses were First Isolated in the Early 1930’s (J Exp Med, 1931) [MEDLINE] (J Exp Med, 1946) [MEDLINE]
- Secondary Bacterial Pneumonia Complicated Nearly All Influenza Deaths in the 1918 Influenza Pandemic and Up to 34% of 2009 Pandemic Influenza A (H1N1) Infections Managed in intensive Care Units Worldwide (JAMA, 2013) [MEDLINE]
- Secondary Bacterial Pneumonia Particularly Contributes to Morbidity/Mortality in Patients >65 y/o (JAMA, 2013) [MEDLINE]
- Secondary Bacterial Pneumonia is Likely More Common than Primary Influenza Pneumonia
- Meta-Analyses Suggest that the Mean Incidence of Secondary Bacterial Pneumonia is 23% of Cases (with a Wide Range: 2-65%) (Influenza Other Respir Viruses, 2016) [MEDLINE]
- Excluding the 7 Studies Which Accounted for 50% of the Heterogeneity in the Data, the Remaining 20 Studies (Representing 64% of All of the Patients) Yielded a Range Between 11-35%
- There Were No Specific Characteristics of the Studies Which Were Associated with Variability in the Coinfection Frequency
- Although Heterogeneity was Greater in the Pediatric Studies, No Significant Trend was Observed when Stratifying the Studies by Mean/Median Age
- Meta-Analyses Suggest that the Mean Incidence of Secondary Bacterial Pneumonia is 23% of Cases (with a Wide Range: 2-65%) (Influenza Other Respir Viruses, 2016) [MEDLINE]
- Microbiology (JAMA, 2013) [MEDLINE]
- General Comments
- In a Meta-Analysis (27 Studies with 3215 Patients), the Most Common Post-Influenza Bacterial Pneumonia Pathogens Have Been Reported to Be Streptococcus Pneumonia (35% of Cases) and Staphylococcus Aureus (28% of Cases) (Influenza Other Respir Viruses, 2016) [MEDLINE]
- Escherichia Coli (see Escherichia Coli)
- Less Common Post-Influenza Bacterial Pneumonia Pathogen
- Haemophilus Influenzae (see Haemophilus Influenzae)
- Less Common Post-Influenza Bacterial Pneumonia Pathogen
- Haemophilus Influenzae was First Described in 1892 by Richard Pfeiffer During an Influenza Pandemic
- Haemophilus Influenzae was Argued by Some to Be the Cause of Influenza Until 1933, When the Viral Nature of Influenza was Firmly Established
- Klebsiella Pneumoniae (see Klebsiella Pneumoniae)
- Less Common Post-Influenza Bacterial Pneumonia Pathogen
- Moraxella Catarrhalis (see Moraxella Catarrhalis)
- Less Common Post-Influenza Bacterial Pneumonia Pathogen
- Pseudomonas Aeruginosa (see Pseudomonas Aeruginosa)
- Less Common Post-Influenza Bacterial Pneumonia Pathogen
- Staphylococcus Aureus (see Staphylococcus Aureus)
- Accounts for 28% of Post-Influenza Bacterial Pneumonia Cases (Influenza Other Respir Viruses, 2016) [MEDLINE]
- Community-Acquired Staphylococcus Aureus Pneumonia is Usually a Post-Influenza Pneumonia (see Influenza Virus) (Clin Infect Dis, 2005) [MEDLINE] (Emerg Infect Dis, 2006) [MEDLINE] (MMWR Morb Mortal Wkly Rep, 2007) [MEDLINE] (Ann Emerg
- Severe Outbreaks of Community-Associated Methicillin-Resistant Staphylococcus Aureus (CA-MRSA) Have Been Reported in Association with Influenza in Previously Healthy Patients
- During the 2003-2004 Influenza Season, 17 Cases of Staphylococcus Aureus Community-Acquired Pneumonia were Reported from 9 States (Emerg Infect Dis, 2006) [MEDLINE]
- During the January, 2007, 10 Cases of Severe Methicillin-Resistant Staphylococcus Aureus Community-Acquired Pneumonia were Reported (MMWR Morb Mortal Wkly Rep. 2007) [MEDLINE]
- During the 2006-2007 Influenza Season, 51 Cases of Staphylococcus Aureus Pneumonia (79% of Cases were MRSA) were Reported from 19 States (Ann Emerg Med, 2009) [MEDLINE]
- 44% of These Cases Had No Known Significant Past Medical History
- 51% of Cases with Staphylococcus Aureus Pneumonia (For Whom Outcome was Known) Died a Median of 4 Days After Symptom Onset
- Only 43% of Cases with Community-Associated MRSA (CA-MRSA) were Empirically Treated with Antibiotics Recommended for MRSA Pneumonia
- Streptococcus Pneumoniae (see Streptococcus Pneumoniae)
- Accounts for 35% of Post-Influenza Bacterial Pneumonia Cases (Influenza Other Respir Viruses, 2016) [MEDLINE]
- Streptococcus Pyogenes (see Streptococcus Pyogenes)
- General Comments
- Physiology
- General
- Influenza Virus Damages Tracheobronchial Epithelium with Loss of Cilia and Decrease in Cell Size
- Streptococcus Pneumoniae
- In a Mouse Model of Influenza and Streptococcus Pneumoniae Infection, the Level of Neuraminidase Activity of Correlated with Increased Adherence and Invasion of Streptococcus Pneumoniae ( J Infect Dis, 2005) [MEDLINE]
- In a Mouse Model, Neuraminidase Cleaved Sialic Acid from Host Cell Glycoconjugates and the Resulting Free Sugar Increased the Growth and Density of Nasopharyngeal Streptococcus Pneumoniae Colonies (Cell Host Microbe, 2014) [MEDLINE]
- Influenza Virus Has Been Demonstrated to Enhance the Attachment of Streptococcus Pneumoniae to Nasopharyngeal Epithelial Cells, Enhancing Colonization (J Infect Dis, 2014) [MEDLINE]
- General
- Diagnosis
- Chest X-Ray/Chest CT (see Chest X-Ray and Chest Computed Tomography)
- Consolidation
- Chest X-Ray/Chest CT (see Chest X-Ray and Chest Computed Tomography)
- Clinical
- Secondary Bacterial Pneumonia Classically Occurs After an Initial Period of Clinical Improvement from the Influenza Virus Infection
- Fever May Decrease After a Few Days into the Influenza Course, But Then Relapses with the Occurrence of Higher Fevers, Cough, Productive Cough (with Sputum), and Radiographic Pulmonary Infiltrates
- Secondary Bacterial Pneumonia Classically Occurs After an Initial Period of Clinical Improvement from the Influenza Virus Infection
Pneumothorax (see Pneumothorax)
Rheumatologic Manifestations
- Myalgias (see Myalgias)
- Epidemiology
- Prominent Feature
- Epidemiology
- Viral Myositis/Rhabdomyolysis (see Viral Myositis and Rhabdomyolysis)
- Epidemiology
- Uncommon
- May Occur in School-Aged Children/Adults/Elderly
- Physiology
- Presence of Influenza Virus in Muscles
- Clinical
- Muscle Tenderness (Especially in the Lower Extremities)
- In Severe Cases, Myositis May Result in Markedly Elevated Creative Kinase (CK) with Myoglobinuria and Acute Kidney Injury (AKI)
- Epidemiology
Pregnancy-Related Manifestations
- Fetal Loss
- Premature Labor
Prevention and Infection Control
Annual Influenza Vaccination
General Comments
- Annual Influenza Vaccination is Recommended for All Patients ≥6 mo Who Do Not Have Contraindications
- Annual Influenza Vaccination Should Occur Before the Onset of Seasonal Influenza Activity in the Community: vaccination should continue to be offered as long as there are influenza viruses circulating in the community
- Clinical Efficacy
- Systematic Review of Efficacy of Influenza Vaccination in Patients with COPD (BMC Pulm Med. 2017) [MEDLINE]
- Evidence supports a positive benefit-risk ratio for seasonal influenza vaccination in patients with COPD
- XXXXX
- Systematic Review of Efficacy of Influenza Vaccination in Patients with COPD (BMC Pulm Med. 2017) [MEDLINE]
Standard-Dose Quadrivalent Live Attenuated Influenza Vaccine (LAIV4)-Intranasal (FluMist Quadrivalent)
- Pharmacology: live, attenuated vaccine against both influenza A + B
- Prepared in Embryonated Chicken Eggs
- Indications
- Healthy, Non-Pregnant Adults 2-49 y/o and Without High-Risk Medical Conditions
- Children 2-8 y/o Group: there is evidence that nasal vaccine provides better protection in this group
- Healthy, Non-Pregnant Adults 2-49 y/o and Without High-Risk Medical Conditions
- Contraindications
- Children 2-4 y/o with History of Wheezing/Asthma in Past 12 mo
- Concomitant Aspirin Use in Children and Adolescents: due to risk of Reye syndrome
- Egg Allergy
- Immunosuppression
- Pregnancy
- Use of Oral Influenza Antiviral Medication in Past 48 hrs
- Not Recommended for Use During the 2016-2017 Influenza Season
- Administration
- Intranasal
- Precautions: immunosuppressed patients should avoid contact with patients vaccinated with the LAIV4 vaccine for at least 7 days after receipt of the vaccine (due to the theoretical risk of influenza transmission)
Standard-Dose Inactivated Influenza Vaccine, Trivalent (IIV3)/Quadrivalent (IIV4) (Multiple Brands)
- Pharmacology: standard-dose inactivated vaccine against both influenza A + B
- Prepared in Embryonated Chicken Egs
- Indications
- Patients ≥6 mo Old
- Contraindications
- Egg Allergy
- Administration
- Intramuscular (IM)
Standard-Dose Inactivated Influenza Vaccine, Trivalent (ccIIV3) (Flucelvax)
- Pharmacology: standard-dose inactivated vaccine against both influenza A + B
- Prepared in Cell Culture
- Indications
- Patients ≥18 y/o
- Administration
- Intramuscular (IM)
Standard-Dose Recombinant Influenza Vaccine, Trivalent (RIV3) (Flublok)
- Pharmacology: standard-dose inactivated vaccine against both influenza A + B
- Prepared Using a Recombinant Baculovirus Expression System
- Indications
- Patients ≥18 yo/
- Administration
- Intramuscular (IM)
High-Dose Inactivated Influenza Vaccine, Trivalent (IIV3) (Fluzone High Dose)
- Pharmacology: high-dose inactivated vaccine against both influenza A + B
- Indications
- Patients ≥65 y/o
- Contraindications
- Egg Allergy
- Administration
- Intramuscular (IM)
Vaccination in Patients with Egg Sensitivity/Allergy
- Egg Allergy Consists of Only Hives
- Either Inactivated Influenza Vaccine (IIV) (Egg or Cell Culture-Based) or Recombinant Influenza Vaccine, Trivalent (RIV3) Vaccine May Be Administered: patient should be observed for ≥30 min after vaccination
- Egg Allergy with History of Angioedema/Respiratory Distress/Lightheadedness/Recurrent Emesis
- Recombinant Influenza Vaccine, Trivalent (RIV3) Vaccine May Be Administered in Patients ≥18 y/o if There are No Other Contraindications
- Egg Sensitivity with Ability to Eat Lightly-Cooked (Scrambled) Eggs without Reaction: these patients are unlikely to be allergic
Preexposure Prophylaxis
Recommendations for Preexposure Prophylaxis (Antiviral Chemoprophylaxis to Prevent Influenza in the Absence of Exposure or an Institutional Outbreak) (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- Antiviral Drugs Should Not Be Used for Routine or Widespread Chemoprophylaxis Outside of Institutional Outbreaks (However, Antiviral Chemoprophylaxis Can Be Considered in Certain Situations)
- Clinicians Can Consider Antiviral Chemoprophylaxis for the Duration of the Influenza Season for Adults and Children Age ≥3 mos Who are at Very High Risk of Developing Complications from Influenza and for Whom Influenza Vaccination is Contraindicated, Unavailable, or Expected to Have Low Effectiveness (e.g. Patients Who are Severely Immunocompromised) (C-II Recommendation)
- Clinicians Can Consider Antiviral Chemoprophylaxis for the Duration of the Influenza Season for Adults and Children Age ≥3 mos Who Have the Highest Risk of Influenza-Associated Complications, Such as Recipients of Hematopoietic Stem Cell Transplant in the First 6–12 mos Posttransplant and Lung Transplant Recipients (B-II Recommendation)
- Clinicians Can Consider Short-Term Antiviral Chemoprophylaxis in Conjunction with Prompt Administration of Inactivated Influenza Vaccine for Unvaccinated Adults and Children Age ≥3 mos Who are at High Risk of Developing Complications from Influenza in Whom Influenza Vaccination is Expected to Be Effective (But Not Yet Administered) When Influenza Activity Has Been Detected in the Community (C-II Recommendation)
- Clinicians Can Consider Short-Term Antiviral Chemoprophylaxis for Unvaccinated Adults, Including Healthcare Personnel, and for Children Aged ≥3 mos Who are in Close Contact with Persons at High Risk of Developing Influenza Complications During Periods of Influenza Activity when Influenza Vaccination is Contraindicated or Unavailable and These High-Risk Patients are Unable to Take Antiviral Chemoprophylaxis (C-III Recommendation)
- Clinicians Can Consider Educating Patients and Parents of Patients to Arrange for Early Empiric Initiation of Antiviral Treatment as an Alternative to Antiviral Chemoprophylaxis (C-III)
- Clinicians Should Use a Neuraminidase Inhibitor (Oral Oseltamivir or Inhaled Zanamivir) if Preexposure Chemoprophylaxis for Influenza is Administered Rather than an Adamantane Antiviral (A-II Recommendation)
- Clinicians Should Administer Preexposure Antiviral Chemoprophylaxis for Adults and Children Aged ≥3 mos Who are at Very High Risk of Developing Complications from Influenza (e.g. Severely Immunocompromised Patients, Such as Hematopoietic Stem Cell Transplant Recipients) for Whom Influenza Vaccination is Contraindicated, Unavailable, or Expected to Have Low Effectiveness, as Soon as Influenza Activity is Detected in the Community and Continued for the Duration of Community Influenza Activity (A-II Recommendation)
- Clinicians Should Test for Influenza and Switch to Antiviral Treatment Dosing in Persons Receiving Preexposure Antiviral Chemoprophylaxis Who Become Symptomatic, Preferably with an Antiviral Drug with a Different Resistance Profile if Not Contraindicated (A-II Recommendation)
Post-Influenza Exposure Prophylaxis
General Comments
- Consists of Treatment of a Patient Following Contact with a Person with Influenza
Regimens
- Influenza Vaccine + Consider Antivirals x 2 wks Following Exposure
- XXXX
- Baloxavir Marboxil (Xofluza) (see Baloxavir Marboxil)
- FDA-Approved for This Indication in Patients ≥12 y/o
- Clinical Efficacy
- Trial of Baloxavir Marboxil for Prophylaxis against Influenza in Household Contacts (NEJM, 2020) [MEDLINE]
- Mechanism: influenza virus CAP-dependent endonuclease inhibitor
- Active Against Influenza A and B Viruses
- Dose: xxx
- xxxx
Recommendations for Postexposure Prophylaxis in Non-Institutional Setting (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- Clinicians Can Consider Postexposure Antiviral Chemoprophylaxis for Asymptomatic Adults and Children Age ≥3 mos Who are at Very High Risk of Developing Complications from Influenza (eg, Severely Immunocompromised Patients) and for Whom Influenza Vaccination is Contraindicated, Unavailable, or Expected to Have Low Effectiveness, After Household Exposure to Influenza (C-II Recommendation)
- Clinicians Can Consider Postexposure Antiviral Chemoprophylaxis (in Conjunction with Influenza Vaccination) for Adults and Children Age ≥3 mos Who are Unvaccinated and are Household Contacts of a Person at Very High Risk of Developing Complications from Influenza (eg, Severely Immunocompromised Persons), After Exposure to Influenza (C-II Recommendation)
- Clinicians Can Consider Educating Patients and Arranging for Early Empiric Initiation of Antiviral Treatment as an Alternative to Postexposure Antiviral Chemoprophylaxis (C-III Recommendation)
- If Chemoprophylaxis is Given, Clinicians Should Administer Postexposure Antiviral Chemoprophylaxis as soon as possible after exposure, Ideally No Later than 48 hrs After Exposure (A-III Recommendation)
- Clinicians Should Not Administer Once-Daily Postexposure Antiviral Chemoprophylaxis if >48 hrs Has Elapsed Since Exposure
- Full-Dose Empiric Antiviral treatment Should Be Initiated as Soon as Symptoms Occur, if Treatment is Indicated (A-III Recommendation)
- Clinicians Should Administer Postexposure Antiviral Chemoprophylaxis in a Nonoutbreak Setting for 7 Days After the Most Recent Exposure to a Close Contact with Influenza (A-III Recommendation)
- Clinicians Should Test for Influenza and Switch to Antiviral Treatment Dosing in Persons Receiving Postexposure Antiviral Chemoprophylaxis Who Become Symptomatic, Preferably with an Antiviral Drug with a Different Resistance Profile if Not Contraindicated (A-III Recommendation)
- Clinicians Should Administer an Neuraminidase Inhibitor ( Oral Oseltamivir of Inhaled Zanamivir) if Postexposure Chemoprophylaxis for Influenza is Given, Rather than an Adamantane Antiviral (A-II Recommendation)
Influenza Infection Control
Droplet Precautions/Isolation
- Half of Health Care Worker Seroconversion is Due to Workplace Exposure and the Other Half is Due to Community Exposure to Virus
- Randomized Effectiveness Trial Comparing Surgical Masks to N95 Respirators in the 2008 Season Demonstrated that Surgical Masks were Equally Efficacious (JAMA, 2009) [MEDLINE]
- Influenza Infection Occurred in 23.6% of Nurses Wearing Regular Masks, as Compared to 22.9% Wearing the N95 Masks
Recommendations for Control of Institutional Influenza Outbreak (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- When Is There Sufficient Evidence of an Influenza Outbreak in a Long-Term Care Facility or Hospital to Trigger Implementation of Control Measures Among Exposed Residents or Patients and Healthcare Personnel to Prevent Additional Cases of Influenza?
- Active Surveillance for Additional Cases Should Be Implemented as Soon as Possible When One Healthcare-Associated Laboratory-Confirmed Influenza Case is Identified in a Hospital or One Case of Laboratory-Confirmed Influenza is Identified in a Long-Term Care Facility (A-III Recommendation)
- Outbreak Control Measures Should Be Implemented as Soon as Possible, Including Antiviral Chemoprophylaxis of Residents/Patients, and Active Surveillance for New Cases, When 2 Cases of Healthcare-Associated Laboratory-Confirmed Influenza are Identified within 72 hrs of Each Other in Residents or Patients of the Same Ward or Unit (A-III Recommendation)
- Implementation of Outbreak Control Measures Can Be Considered as Soon as Possible if One or More Residents or Patients Has Suspected Healthcare-Associated Influenza and Results of Influenza Molecular Testing are Not Available on the Day of Specimen Collection (B-III Recommendation)
- Which Residents/Patients Should Be Considered to Have Influenza and Be Treated With Antivirals During an Influenza Outbreak in a Long-term Care Facility or Hospital?
- When an Influenza Outbreak Has Been Identified in a Long-Term Care Facility or Hospital, Influenza Testing Should Be Done for Any Resident/Patient with One or More Acute Respiratory Symptoms, with or without Fever, or Any of the Following without Respiratory Symptoms (Temperature Elevation or Reduction, or Behavioral Change) (A-III Recommendation)
- Empiric Antiviral Treatment Should Be Administered as Soon as Possible to Any Resident or Patient with Suspected Influenza During an Influenza Outbreak without Waiting for the Results of Influenza Diagnostic Testing (A-III Recommendation)
- To Control an Influenza Outbreak in a Long-term Care Facility or Hospital, Should Antiviral Chemoprophylaxis Be Administered to Exposed Residents/Patients?
- Antiviral Chemoprophylaxis Should Be Administered as Soon as Possible to All Exposed Residents or Patients Who Do Not Have Suspected or Laboratory-Confirmed Influenza Regardless of Influenza Vaccination History, in Addition to Implementation of All Other Recommended Influenza Outbreak Control Measures, When an influenza Outbreak Has Been Identified in a Long-Term Care Facility or Hospital (A-III Recommendation)
- During an Influenza Outbreak at a Long-term Care Facility, Should Antiviral Chemoprophylaxis Be Administered to Residents Only on Affected Units or to All Residents in the Facility?
- Antiviral Chemoprophylaxis Should Be Administered to Residents on Outbreak-Affected Units, in Addition to Implementing Active Daily Surveillance for New Influenza Cases Throughout the Facility (A-II Recommendation)
- Which Healthcare Personnel Should Receive Antiviral Chemoprophylaxis During an Institutional Outbreak?
- Clinicians Can Consider Antiviral Chemoprophylaxis for Unvaccinated Staff, Including Those for Whom Chemoprophylaxis May Be Indicated Based Upon Underlying Conditions of the Staff or Their Household Members for the Duration of the Outbreak (C-III Recommendation)
- Clinicians Can Consider Antiviral Chemoprophylaxis for Staff Who Receive Inactivated Influenza Vaccine During an Institutional Influenza Outbreak for 14 Days Postvaccination (C-III Recommendation)
- Clinicians Can Consider Antiviral Chemoprophylaxis for Staff Regardless of Influenza Vaccination Status to Reduce the Risk of Short Staffing in Facilities and Wards Where Clinical Staff are Limited and to Reduce Staff Reluctance to Care for Patients with Suspected Influenza (C-III Recommendation)
- How Long Should Antiviral Chemoprophylaxis Be Given to Residents During an Influenza Outbreak in a Long-term Care Facility?
- Clinicians should administer Antiviral Chemoprophylaxis for 14 Days and Continue for at Least 7 Days After the Onset of Symptoms in theLast Case Identified During an Institutional Influenza Outbreak (A-III Recommendation)
Treatment
Antiviral Agents
Clinical Efficacy
- Study of Antiviral Therapy and Outcome of Influenza Requiring Hospitalization in Ontario, Canada (Clin Infect Dis, 2007) [MEDLINE]
- Observational Study Which Suggested that Treatment with Oseltamivir was Associated with Decreased Mortality Rate in Patients Hospitalized for Concomitant Influenza and Community-Acquired Pneumonia
- Study of the Outcomes of Patients Hospitalized with Severe Influenza (Thorax, 2010) [MEDLINE]
- Observational Study Which Suggested that Treatment with Oseltamivir was Associated with Decreased Mortality Rate in Patients Hospitalized for Concomitant Influenza and Community-Acquired Pneumonia
- Systematic Review and Meta-Analysis of Influenza Treatments (Annals Int Med, 2012) [MEDLINE]
- In High-Risk Populations, Oral Oseltamivir Decreased Mortality Rate (Odds Ratio: 0.23), Hospitalization (Odds Ratio: 0.75), and Duration of Symptoms (From 45 hrs -> 33 hrs)
- Earlier Treatment with Oseltamivir (Within 48 hrs) was Associated with Better Outcomes
- Inhaled Zanamivir Decreased Symptom Duration (From 28 hrs -> 23 hrs) and Hospitalization (Odds Ratio: 0.66), But Had More Complications
- No Differences Between Oseltamivir and Zanamivir
- Oral Amantadine Decreased Mortality and Pneumonia: data from single study only
- Hong Kong Prospective Study of High-Dose Oseltamivir in Hospitalized Adult Patients with Influenza A + B (Clin Infect Dis, 2013) [MEDLINE]
- No Benefit of High-Dose Oseltamivir in Influenza A
- Improved Virologic Response in Influenza B
- Cochrane Database Systematic Review of Efficacy of Neuraminidase Inhibitors in Influenza (Cochrane Database Syst Rev, 2014) [MEDLINE]
- Time to First Symptom Alleviation
- Oseltamavir Decreased the Time to First Alleviation of Symptoms in Adults by 16.8 hrs (Time to First Alleviation of Symptoms Decreased From 7.0 -> 6.3 Days): effect was observed in healthy children, but no effect was observed in asthmatic children
- Zanamivir Decreased the Time to First Alleviation of Symptoms in Adults by 0.60 days (Mean Duration of Symptoms Decreased From 6.6 -> 6.0 Days): no effect was observed in children
- Hospitalizations
- Oseltamavir Had No Effect on Hospitalization Rate in Adults/Children: prophylaxis also had no no effect on hospitalizations
- Zanamivir Hospitalization Rate was Not Reported
- Serious Influenza Complications
- Oseltamavir Did Not Decrease Serious Influenza Complications in Adults/Children: insufficient data to determine effect of oseltamavir prophylaxis on serious complications
- Zanamivir Did Not Decrease Serious Influenza Complications in Adults: prophylaxis also had no no effect on serious complications
- Pneumonia
- Oseltamavir Decreased the Investigator-Reported, Unverified Pneumonia Rate: however, effect was not significant in trials which used more detailed diagnostic form for pneumonia (and no trials reported effect on radiologically-confirmed pneumonia)
- Oseltamavir Prophylaxis Had No Effect on Self-Reported, Investigator-Mediated, Unverified Pneumonia Rate in Adults
- Zanamivir Had No Effect on Self-Reported or Radiologically-Confirmed Pneumonia
- Zanamivir Prophylaxis Decreased the Self-Reported, Investigator-Mediated, Unverified Pneumonia Rate in Adults
- Bronchitis/Sinusitis/Otitis Media
- Oseltamavir Had No Effect on the Risk of Bronchitis in Adults
- Zanamivir Decreased the Risk of Bronchitis in Adults
- Neither Oseltamavir/Zanamivir Decreased Rates of Sinusitis/Otitis Media in Adults/Children
- Prophylaxis
- Oseltamavir/Zanamivir Prophylaxis Decreased the Risk of Symptomatic Influenza
- Conclusions
- Oseltamavir/Zanamivir Have Small, Non-Specific Effects on Decreasing the Time to Alleviation of Symptoms in Adults, But Not in Asthmatic Children
- Oseltamavir/Zanamivir Prophylaxis Decrease the Risk of Symptomatic Influenza
- Trials Do Not Settle the Question of Whether Influenza Complications of Influenza (Pneumonia) are Reduced, Because of a Lack of Diagnostic Definitions
- The Lower Bioavailability of Zanamivir May Explain its Lower Toxicity, as Compared to Oseltamivir
- Time to First Symptom Alleviation
- Study of High-Dose Oseltamivir in Adults Hospitalized with Influenza A and B (Clin Infect Dis, 2013) [MEDLINE]
- High-Dose Oseltamivir Demonstrated No Benefit in Influenza A, But Improved the Virologic Response in Influenza B
- Systematic Review of Oseltamivir for Influenza in Adults/Children (BMJ, 2014) [MEDLINE]
- In Prophylactic Studies, Oseltamivir Decreases the Proportion of Symptomatic Influenza
- In Treatment Studies. Oseltamivir Also Modestly Decreases the Time to First Alleviation of Symptoms, But it Causes Nausea/Vomiting and Increases the Risk of Headaches and Renal and Psychiatric Syndromes
- The Evidence of Clinically Significant Effects on Complications and Viral Transmission is Limited Because of Rarity of Such Events and Problems with Study Design
- Systematic Review of Zanamivir for Influenza in Adults/Children (BMJ, 2014) [MEDLINE]
- Based on a full assessment of all trials conducted, zanamivir reduces the time to symptomatic improvement in adults (but not in children) with influenza-like illness by just over half a day, although this effect might be attenuated by symptom relief medication
- Zanamivir also reduces the proportion of patients with laboratory confirmed symptomatic influenza
- We found no evidence that zanamivir reduces the risk of complications of influenza, particularly pneumonia, or the risk of hospital admission or death. Its harmful effects were minor (except for bronchospasm), perhaps because of low bioavailability
- Meta Analysis of Oseltamivir in Influenza in Adults (Lancet, 2015) [MEDLINE]
- Oseltamivir in Adults with Influenza Accelerates Time to Clinical Symptom Alleviation, Reduces Risk of Lower Respiratory Tract Complications, and Reduces Risk of Hospital Admission, But Increases the Occurrence of Nausea/Vomiting
- Meta-Analysis of Outpatient Neuraminidase Inhibitor Treatment in Patients Infected With Influenza A(H1N1)pdm09 at High Risk of Hospitalization (Clin Infect Dis, 2017) [MEDLINE]
- The Highest Level of Clinical Benefit was Achieved if the Anti-Influenza Therapy was Received Within 48 hrs After the Onset of Symptoms (JAMA, 2010) [MEDLINE] (Clin Infect Dis, 2010) [MEDLINE]
- However, There May Be a Clinical Benefit of Anti-Influenza Treatment Up to 4-5 Days After Symptom Onset (Thorax, 2010) [MEDLINE] (Clin Infect Dis, 2012) [MEDLINE]
- Open-Label, Pragmatic, Adaptive, Randomized Controlled Trial of Adding Oseltamivir to Usual Care in Patients Age ≥1 y/o Presenting with Influenza-Like Illness in Primary Care (Lancet, 2020) [MEDLINE]
- Primary Care Patients with Influenza-Like illness treated with Oseltamivir Recovered One Day Sooner on Average Than Those Managed by Usual Care Alone
- Older, Sicker Patients with Comorbidities and Longer Previous Symptom Duration Recovered 2-3 Days Sooner
- Systematic Review and Meta-Analysis of Influenza Antivirals (JAMA Netw Open, 2021) [MEDLINE]: n = 11,897 (n= 26 trials)
- All Influenza Antiviral Agents (Neuraminidase Inhibitors: Oseltamivir, Peramivir, Zanamivir, or Laninamivir; or Endonuclease Inhibitor: Baloxavir) were Associated with Shortening Time to Alleviation of Influenza Symptoms
- Zanamivir was Associated with the Shortest Time to Alleviation of Influenza Symptoms
- Baloxavir was Associated with a Decrease in the Rate of Influenza-Related Complications
Recommendations for Initiating Influenza Antiviral Treatment (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
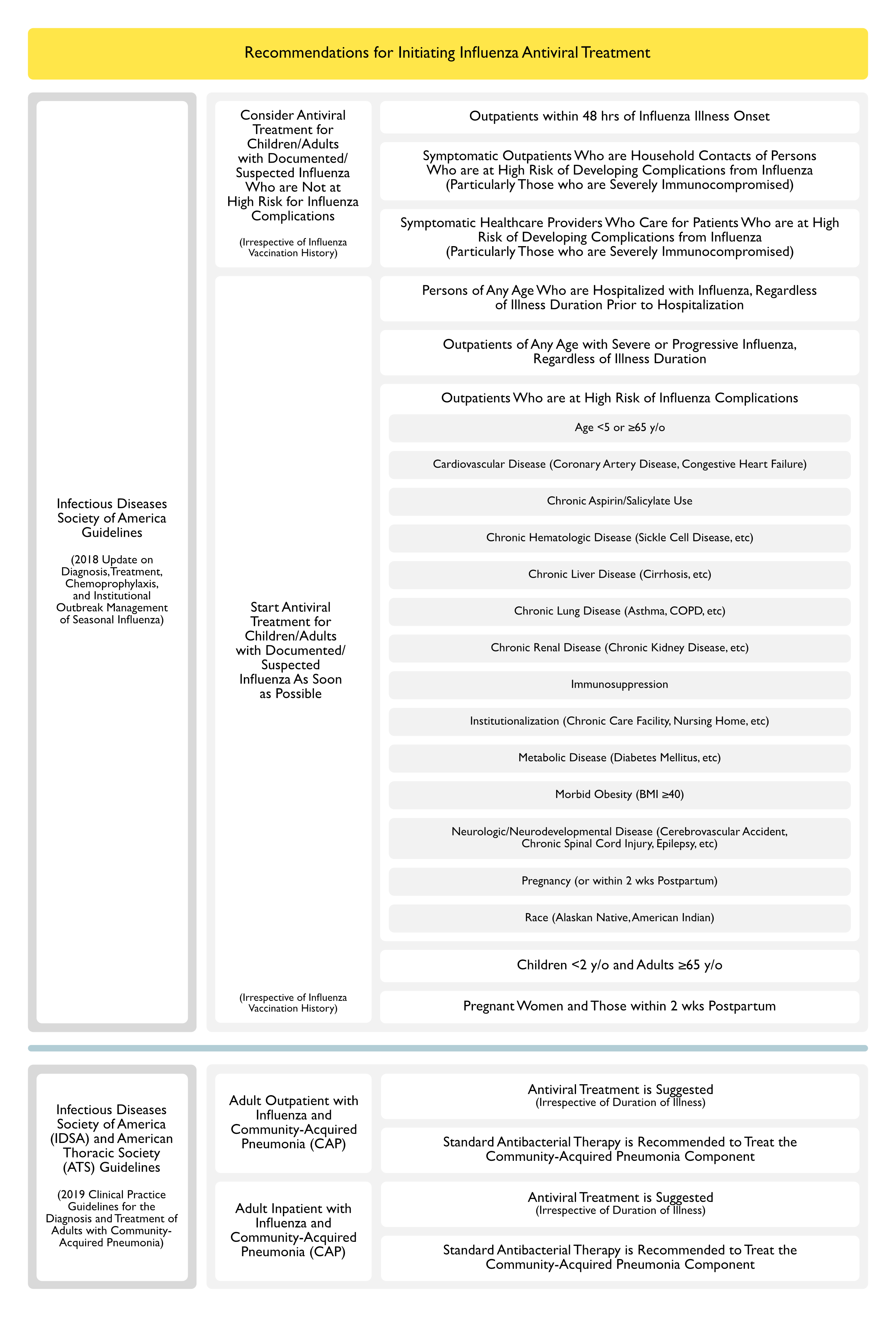
- Consider Antiviral Treatment for Children/Adults with Documented/Suspected Influenza Who are Not at High Risk for Influenza Complications (Irrespective of Influenza Vaccination History)
- Outpatients within 48 hrs of Influenza Illness Onset (C-I Recommendation)
- Symptomatic Outpatients Who are Household Contacts of Persons Who are at High Risk of Developing Complications from Influenza (Particularly Those who are Severely Immunocompromised) (C-III Recommendation)
- Symptomatic Healthcare Providers Who Care for Patients Who are at High Risk of Developing Complications from Influenza (Particularly Those who are Severely Immunocompromised) (C-III Recommendation)
- Start Antiviral Treatment for Children/Adults with Documented/Suspected Influenza As Soon as Possible (Irrespective of Influenza Vaccination History)
- Persons of Any Age Who are Hospitalized with Influenza, Regardless of Illness Duration Prior to Hospitalization (A-II Recommendation)
- Outpatients of Any Age with Severe or Progressive Influenza, Regardless of Illness Duration (A-III Recommendation)
- Outpatients Who are at High Risk of Influenza Complications (A-II Recommendation)
- See Epidemiology Section Above (“Factors Which Predict High Risk for Influenza Complications”) For More Detail
- Age <5 or ≥65 y/o
- Cardiovascular Disease (Coronary Artery Disease, Congestive Heart Failure, etc)
- Chronic Aspirin/Salicylate Use
- Chronic Hematologic Disease (Sickle Cell Disease, etc)
- Chronic Liver Disease (Cirrhosis, etc)
- Chronic Lung Disease (Asthma, COPD, etc)
- Chronic Renal Disease (Chronic Kidney Disease, etc)
- Immunosuppression
- Institutionalization (Chronic Care Facility, Nursing Home, etc)
- Metabolic Disease (Diabetes Mellitus, etc)
- Morbid Obesity (BMI ≥40)
- Neurologic/Neurodevelopmental Disease (Cerebrovascular Accident, Chronic Spinal Cord Injury, Epilepsy, etc)
- Pregnancy (or within 2 wks Postpartum)
- Race (Alaskan Native, American Indian)
- See Epidemiology Section Above (“Factors Which Predict High Risk for Influenza Complications”) For More Detail
- Children <2 y/o and Adults ≥65 y/o (A-III Recommendation)
- Pregnant Women and Those within 2 wks Postpartum (A-III Recommendation)
Recommendations-Treatment of Adults with Concomitant Influenza and Community-Acquired Pneumonia (CAP) (American Thoracic Society and Infectious Diseases Society of America 2019 Clinical Practice Guidelines for the Diagnosis and Treatment of Adults with Community-Acquired Pneumonia) (Am J Respir Crit Care Med, 2019) [MEDLINE]
- Outpatient Setting
- In Adults with Influenza and Community-Acquired Pneumonia, Anti-Influenza Treatment (Such as Oseltamivir) is Suggested, Independent of the Duration of Illness Before Diagnosis (Conditional Recommendation, Low Quality of Evidence)
- In Adults with Concomitant Influenza and Clinical and Radiographic Evidence of Community-Acquired Pneumonia, Standard Antibacterial Therapy is Recommended to Treat the Community-Acquired Pneumonia in Both Outpatient/Inpatient Settings (Strong Recommendation, Low Quality of Evidence)
- Inpatient Setting
- In Adults with Influenza and Community-Acquired Pneumonia, Anti-Influenza Treatment (Such as Oseltamivir) is Recommended, Independent of the Duration of Illness Before Diagnosis (Strong Recommendation, Moderate Quality of Evidence)
- In Adults with Concomitant Influenza and Clinical and Radiographic Evidence of Community-Acquired Pneumonia, Standard Antibacterial Therapy is Recommended to Treat the Community-Acquired Pneumonia in Both Outpatient/Inpatient Settings (Strong Recommendation, Low Quality of Evidence)
Recommendations for Choice of Agent/Duration of Influenza Antiviral Treatment (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- Clinicians Should Start Antiviral Treatment as Soon as Possible with a Single Neuraminidase Inhibitor (Either Oral Oseltamivir, Inhaled Zanamivir, or Intravenous Peramivir) and Not Use a Combination of Neuraminidase Inhibitors (A-1 Recommendation)
- Clinicians Should Not Routinely Use Higher Doses of US FDA–Approved Neuraminidase Inhibitor Drugs for the Treatment of Seasonal Influenza (A-II Recommendation)
- Clinicians Should Treat Uncomplicated Influenza in Otherwise Healthy Ambulatory Patients for 5 Days with Oral Oseltamivir or Inhaled Zanamivir, or a Single Dose of Intravenous Peramivir (A-1 Recommendation)
- Clinicians Can Consider Longer Duration of Antiviral Treatment for Patients with a Documented or Suspected Immunocompromising Condition or Patients Requiring Hospitalization for Severe Lower Respiratory Tract Disease (Especially Pneumonia or Acute Respiratory Distress Syndrome/ARDS), as Influenza Viral Replication is Often Protracted (C-III Recommendation)
- In Patient with Influenza Who Does Not Improve with Antiviral Treatment or Demonstrates Clinical Deterioration During or After Treatment, Clinicians Should Investigate Other Etiologies Besides influenza Virus Infection (A-III Recommendation)
Recommendations for Testing for Infection With an Antiviral-Resistant Influenza Virus (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- Influenza Neuraminidase Inhibitor Resistance Testing Can Be Considered For the Following
- Patients Who Develop Laboratory-Confirmed Influenza While on or Immediately After Neuraminidase Inhibitor Chemoprophylaxis (C-III Recommendation)
- Patients with an Immunocompromising Condition and Evidence of Persistent Influenza Viral Replication (eg, After 7–10 Days, Demonstrated by Persistently Positive RT-PCR or Viral Culture Results) and Remain Ill During or After Neuraminidase Inhibitor Treatment (B-III)
- Patients with Laboratory-Confirmed Influenza who inadvertently received subtherapeutic Neuraminidase Inhibitor dosing (C-III Recommendation)
- Patients with Severe Influenza Who Do Not Improve with Neuraminidase Inhibitor Treatment and Have Evidence of Persistent Influenza Viral Replication (eg, after 7–10 days) (C-II Recommendation)
- Clinicians Should Remain Informed on Current CDC and World Health Organization Surveillance Data on the Frequency and Geographic Distribution of Neuraminidase Inhibitor-Resistant Influenza Viruses During Influenza Season, and with the Latest CDC Antiviral Treatment Recommendations (A-III Recommendation)
Amantadine (Symmetrel, Symadine) (see Amantadine)
- Mechanism
- M2 Ion Channel Blocker (Adamantane): not currently recommended by the CDC for Influenza A (due to resistance) [MEDLINE]
- Activity Against Influenza A Virus
- Cost
- Inexpensive
- Administration
- Oral
- Indications
- Treatment
- Prophylaxis (For Patients >1 y/o)
- Given x 2 wks during epidemic, during which time vaccine will induce antibody response
- Given during facility outbreak, to all exposed regardless of vaccination status, until 1 wk after end of outbreak
- Contraindications: pregnancy
- Metabolism: renal clearance
- Adverse Effects
Rimantadine (Flumadine) (see Rimantadine)
- Mechanism
- M2 Ion Channel Blocker (Adamantane)
- Not Currently Recommended by the CDC for Influenza A Virus (Due to Resistance) [MEDLINE]
- Activity Against Influenza A
- Cost
- Inexpensive
- Administration
- Oral
- Clinical Efficacy
- Early treatment (within 48 hrs) is probably better than later treatment
- Probably decreases the duration of fever and symptoms by 1-2 days
- Indications
- Treatment (For Patients >13 y/o)
- Prophylaxis (For Patients >1 y/o)
- Given x 2 wks during epidemic, during which time vaccine will induce antibody response
- Given during facility outbreak, to all exposed regardless of vaccination status, until 1 wk after end of outbreak
- Contraindications: pregnancy
- Metabolism
- Hepatic Clearance
- Adverse Effects
Oseltamivir (Tamiflu) (see Oseltamivir)
- Pharmacology
- Neuraminidase Inhibitor with Activity Against Influenza A and B Viruses
- Initial FDA Approval for the Treatment of Influenza was in 1999
- Indications
- Treatment (For Patients >1 y/o)
- Prophylaxis (For Patients >13 y/o)
- Given x 2 wks During Epidemic, During Which Time Vaccine Will Induce Antibody Response
- Given During Facility Outbreak, to All Exposed Regardless of Vaccination Status, Until 1 wk After End of Outbreak
- Cost
- More Expensive than Older Agents
- Administration (Oral)
- Prophylaxis (Adult): 75 mg PO BID
- Treatment (Adult): 75 mg PO BID
- Oseltamivir Resistance
- Sporadic Oseltamivir-Resistant 2009 H1N1 Has Been Reported
- Oseltamivir Resistance was Associated with the H275Y (Histidine->Tyrosine) Substitution in Neuraminidase in H1N1 During 2007-2008: this strain was spread worldwide, but has not been prominent in later influenza seasons -> nearly all cases are sensitive to zanamivir
- Adverse Effects
- Acute Kidney Injury (AKI) (see Acute Kidney Injury): unclear association
- Nausea/Vomiting (see Nausea and Vomiting): occurs in 19% of cases (decreased when taken with food)
- Psychiatric Effects: psychiatric adverse effects appear to be dose-related (Cochrane Database Syst Rev, 2014) [MEDLINE]
Zanamivir (Relenza) (see Zanamivir)
- Pharmacology
- Neuraminidase Inhibitor
- Activity Against Influenza A and B Viruses
- Indications
- Treatment (For Patients >7 y/o)
- Prophylaxis
- Cost
- More Expensive than Older Agents
- Administration
- Inhalation (Treatment): 10 mg (2 Inhalations) BID (child >13 y/o and adult)
- Inhalation (Propylaxis): 10 mg (2 Inhalations) qday (child >13 y/o and adult)
- Intravenous (IV)
- Adverse Effects
- Bronchospasm (see Obstructive Lung Disease)
- Associated with the Use of Inhaled Zanamivir
- Use Zanamivir with Caution in Chronic Obstructive Pulmonary Disease (COPD) and Asthma (see Chronic Obstructive Pulmonary Disease and Asthma)
- Bronchospasm (see Obstructive Lung Disease)
Baloxavir Marboxil (Xofluza) (see Baloxavir Marboxil)
- Pharmacology
- Influenza Virus CAP-Dependent Endonuclease Inhibitor
- Active Against Influenza A and B Viruses
- Indications
- XXXX
- Cost
- XXXXX
- Administration (Oral)
- XXXXXXXXX
- Adverse Effects
- XXXXXXXX
Antibacterial Agents
Recommendations for Investigating and Treating Bacterial Coinfection of the Upper/Lower Respiratory Tract (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- Clinicians Should Investigate and Empirically Treat Bacterial Coinfection in Patients with Suspected/Confirmed Influenza Who Present Initially with Severe Disease (Extensive Pneumonia, Respiratory Failure, Hypotension, and Fever), in Addition to Antiviral Treatment for Influenza (A-II Recommendation)
- Clinicians Should Investigate and Empirically Treat Bacterial Coinfection in Patients Who Deteriorate After Initial Improvement, Particularly in Those Treated with Influenza Antivirals (A-III Recommendation)
- Clinicians Can Consider Investigating Bacterial Coinfection in Patients Who Fail to Improve After 3–5 Days of Influenza Antiviral Treatment (C-III Recommendation)
- In Patient with Influenza Who Does Not Improve with Antiviral Treatment or Demonstrates Clinical Deterioration During or After Treatment, Clinicians Should Investigate Other Causes Besides Influenza Virus Infection in Influenza Patients Who Fail to Improve or Deteriorate Despite Antiviral Treatment (A-III Recommendation)
Corticosteroids (see Corticosteroids)
Recommendations for Adjunctive Therapies in Influenza (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- Clinicians Should Not Administer Corticosteroid Adjunctive Therapy for the Treatment of Adults/Children with Suspected/Confirmed Seasonal Influenza, Influenza-Associated Pneumonia, Respiratory Failure, or Acute Respiratory Distress Syndrome (ARDS), Unless Clinically Indicated for Other Reasons (A-III Recommendation)
Recommendations (American Thoracic Society and Infectious Diseases Society of America 2019 Clinical Practice Guidelines for the Diagnosis and Treatment of Adults with Community-Acquired Pneumonia) (Am J Respir Crit Care Med, 2019) [MEDLINE]
- Routine Use of Corticosteroids is Not Recommended in Adults with Non-Severe Community-Acquired Pneumonia (Strong Recommendation, High Quality of Evidence) or Severe Community-Acquired Pneumonia (Conditional Recommendation, Moderate Quality of Evidence)
- Routine Use of Corticosteroids is Not Recommended in Adults with Severe Influenza-Related Pneumonia (Conditional Recommendation, Low Quality of Evidence)
- In the Setting of Refractory Septic Shock, Surviving Sepsis Recommendations for the Use of Corticosteroids are Recommended
Intravenous Immunoglobulin (see Intravenous Immunoglobulin)
Recommendations for Adjunctive Therapies in Influenza (Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza) (Clin Infect Dis, 2019) [MEDLINE]
- Clinicians Should Not Routinely Administer Immunomodulation Using Immunoglobulin Preparations Such as Intravenous Immunoglobulin for Treatment of Adults/Children with Suspected/Confirmed Seasonal Influenza (A-III Recommendation)
Prognosis
Natural History/Prognosis
- Disease Course: most cases of influenza are self-limited
Epidemiology of Avian Influenza H7N9 Infection in China [MEDLINE]
- Mortality Rate: 34% (with median duration of illness: 21 days)
General Prognosis
- Mortality from Influenza Disproportionately Affects the Elderly ( JAMA, 2003) [MEDLINE] Annual estimates of influenza-associated deaths increased significantly between the 1976-1977 and 1998-1999 seasons for all 3 death categories (P<.001 for each category). For the 1990-1991 through 1998-1999 seasons, the greatest mean numbers of deaths were associated with influenza A(H3N2) viruses, followed by RSV, influenza B, and influenza A(H1N1). Influenza viruses and RSV, respectively, were associated with annual means (SD) of 8097 (3084) and 2707 (196) underlying pneumonia and influenza deaths, 36 155 (11 055) and 11 321 (668) underlying respiratory and circulatory deaths, and 51 203 (15 081) and 17 358 (1086) all-cause deaths. For underlying respiratory and circulatory deaths, 90% of influenza- and 78% of RSV-associated deaths occurred among persons aged 65 years or older. Influenza was associated with more deaths than RSV in all age groups except for children younger than 1 year. On average, influenza was associated with 3 times as many deaths as RSV.
- Influenza deaths have increased substantially in the last 2 decades, in part because of aging of the population, underscoring the need for better prevention measures, including more effective vaccines and vaccination programs for elderly persons
Hospitalization Rates
- Study of Hospitalization Rates for Pneumonia in Patients >65 y/o in the US from 1988-2002 (JAMA, 2005) [MEDLINE]
- RESULTS
- Hospitalization rates by both first-listed and any-listed discharge codes for pneumonia increased by 20% from 1988-1990 to 2000-2002 for patients aged 65 to 74 years (P = .01) and for patients aged 75 to 84 years (P<.001)
- Rates of hospitalization for pneumonia were 2-fold higher for patients aged 85 years or older (51 per 1000 population for first-listed discharge code of pneumonia; 95% confidence interval [CI], 46-55 per 1000 population) than among patients aged 75 to 84 years (26 per 1000 population; 95% CI, 24-28 per 1000 population), but did not significantly increase from 1988-1990 to 2000-2002
- The proportion of patients aged 65 years or older diagnosed with pneumonia and a chronic cardiac disease, chronic pulmonary disease, or diabetes mellitus increased from 66% (SE, 1.0%) in 1988-1990 to 77% (SE, 0.8%) in 2000-2002. The risk of death during a hospitalization for pneumonia compared with the risk of death during a hospital stay for the 10 other most frequent causes of hospitalization was 1.5 (95% CI, 1.4-1.7) and remained constant from 1988-1990 to 2000-2002
- CONCLUSIONS
- Hospitalization rates for pneumonia have increased among US adults aged 64 to 74 years and aged 75 to 84 years during the past 15 years
- Among those aged 85 years or older, at least 1 in 20 patients were hospitalized each year due to pneumonia
- Concomitantly, the proportion of comorbid chronic diseases has increased
- Efforts to prevent pneumonia should include reducing preventable comorbid conditions and improving vaccine effectiveness and vaccination programs in elderly persons.
- RESULTS
References
General
- Toxic shock syndrome complicating influenza A in a child: case report and review. Clin Infect Dis. 1993;17(1):43 [MEDLINE]
- Cardiac findings during uncomplicated acute influenza in ambulatory adults. Clin Infect Dis. 2005;40(3):415 [MEDLINE]
- Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775 [MEDLINE]
- Preparing for the sickest patients with 2009 influenza A (H1N1). JAMA, Published online October 12, 2009 [MEDLINE]
- Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A (H1N1) infection in Canada [published online October 12, 2009]. JAMA doi:10.1001/jama.2009.1496 [MEDLINE]
- Critically ill patients with 2009 influenza A (H1N1) in Mexico [published online October 12, 2009]. JAMA. doi:10.1001/jama.2009.1536 [MEDLINE]
- Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial [published online October 1, 2009]. JAMA doi:10.1001/jama.2009.1466 [MEDLINE]
- Seasonal Influenza in Adults and Children— Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management: Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009 Apr 15;48(8):1003-32. doi: 10.1086/598513 [MEDLINE]
- Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(1):1–24 [MEDLINE]
- Transocular entry of seasonal influenza-attenuated virus aerosols and the efficacy of n95 respirators, surgical masks, and eye protection in humans. J Infect Dis. 2011;204(2):193 [MEDLINE]
- Accuracy of Rapid Influenza Diagnostic Tests: A Meta-analysis. Ann Intern Med. 2012;156:500–511 [MEDLINE]
- Epidemiology of human infections with avian influenza A (H7N9) virus in China. N Engl J Med 2014;370:520-532 [MEDLINE]
- Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015 Aug 7;64(30):818-25 [MEDLINE]
- Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis. 2019;68(6):e1 [MEDLINE]
Epidemiology
- Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013 Aug 23;347:f5061. doi: 10.1136/bmj.f5061 [MEDLINE]
- Seasonal influenza vaccination in patients with COPD: a systematic literature review. BMC Pulm Med. 2017 May 3;17(1):79. doi: 10.1186/s12890-017-0420-8 [MEDLINE]
- Influenza vaccine for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018 Jun 26;6:CD002733. doi: 10.1002/14651858.CD002733.pub3 [MEDLINE]
- Cigarette smoking and the occurrence of influenza – Systematic review. J Infect. 2019 Nov;79(5):401-406. doi: 10.1016/j.jinf.2019.08.014 [MEDLINE]
- Risk of Severe Influenza Among Adults With Chronic Medical Conditions. J Infect Dis. 2020 Jan 2;221(2):183-190. doi: 10.1093/infdis/jiz570 [MEDLINE]
Physiology
- Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J. 2016 Mar;47(3):954-66. doi: 10.1183/13993003.01282-2015 [MEDLINE]
- Influenza A Virus Infection Triggers Pyroptosis and Apoptosis of Respiratory Epithelial Cells through the Type I Interferon Signaling Pathway in a Mutually Exclusive Manner. J Virol. 2018 Jun 29;92(14):e00396-18 [MEDLINE]
- How the Respiratory Epithelium Senses and Reacts to Influenza Virus. Am J Respir Cell Mol Biol. 2019 Mar;60(3):259-268. doi: 10.1165/rcmb.2018-0247TR [MEDLINE]
Clinical
General
- Swine influenza: II, a hemophilic bacillus from the respiratory tract of infected swine. J Exp Med. 1931;54(3):361-371 [MEDLINE]
- Asian influenza A in Boston, 1957-1958. I. Observations in thirty-two influenza-associated fatal cases. AMA Arch Intern Med. 1959;103(4):515 [MEDLINE]
- Pulmonary mechanics after uncomplicated influenza A infection. Am Rev Respir Dis. 1976;113(2):141 [MEDLINE]
- Neurophysiology of the cough reflex. Eur Resp J 1995; 8: 1193–202 [MEDLINE]
- Influenza A outbreak among adolescents in a ski hostel. Eur J Clin Microbiol Infect Dis. 1998;17(2):128 [MEDLINE]
- Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000; 160: 3243–47 [MEDLINE]
Acute Respiratory Distress Syndrome (ARDS) (see Acute Respiratory Distress Syndrome)
- Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896 [MEDLINE]
- Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935 [MEDLINE]
- Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680 [MEDLINE]
- Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872 [MEDLINE]
- Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361(7):674 [MEDLINE]
- Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302(17):1880 [MEDLINE]
- Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888 [MEDLINE]
- Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362(18):1708 [MEDLINE]
- Novel influenza A (H1N1) infection: chest CT findings from 21 cases in Seoul, Korea. Clin Radiol. 2011 Feb;66(2):118-24. doi: 10.1016/j.crad.2010.07.011 [MEDLINE]
- Recurrent Spontaneous Pneumothorax during the Recovery Phase of ARDS Due to H1N1 Infection. Balkan Med J. 2013 Mar;30(1):123-5. doi: 10.5152/balkanmedj.2012.086 [MEDLINE]
- Critically ill patients with influenza A(H1N1)pdm09 virus infection in 2014. JAMA. 2014;311(13):1289 [MEDLINE]
Pneumonia (see Community-Acquired Pneumonia)
- Sulfonamide chemotherapy of combined infection with influenza virus and bacteria. J Exp Med. 1946;83: 505-518 [MEDLINE]
- Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40(1):100 [MEDLINE]
- Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis. 2005;192(2):249 [MEDLINE]
- Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis. 2006;12(6):894 [MEDLINE]
- Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza–Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep. 2007;56(14):325 [MEDLINE]
- Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. 2009;53(3):358 [MEDLINE]
- Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309(3):275 [MEDLINE]
- How important is methicillin-resistant Staphylococcus aureus as a cause of community-acquired pneumonia and what is best antimicrobial therapy? Infect Dis Clin North Am. 2013 Mar;27(1):177-88 [MEDLINE]
- High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis. 2014 Nov 15;210(10):1649-57. doi: 10.1093/infdis/jiu326. Epub 2014 Jun 6 [MEDLINE]
- Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16(1):55 [MEDLINE]
- The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10(5):394 [MEDLINE]
Invasive Pulmonary Aspergillosis (see Invasive Pulmonary Aspergillosis)
- Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782 [MEDLINE]
- Influenza-Associated Pulmonary Aspergillosis: A Local or Global Lethal Combination? Clin Infect Dis. 2020;71(7):1764 [MEDLINE]
- High Rates of Influenza-Associated Invasive Pulmonary Aspergillosis May Not Be Universal: A Retrospective Cohort Study from Alberta, Canada. Clin Infect Dis. 2020;71(7):1760 [MEDLINE]
- Influenza-Associated Pulmonary Aspergillosis: A Local or Global Lethal Combination? Clin Infect Dis. 2020;71(7):1764 [MEDLINE]
Necrotizing Tracheobronchitis (see Acute Bronchitis)
- Fatal influenza A infection with Staphylococcus aureus superinfection in a 49-year-old woman presenting as sudden death. Int J Legal Med. 2005 Jan;119(1):40-3. doi: 10.1007/s00414-004-0472-1 [MEDLINE]
- A Case of Severe Pseudomembranous Tracheobronchitis Complicated by Co-infection of Influenza A (H1N1) and Staphylococcus aureus in an Immunocompetent Patient. Tuberc Respir Dis (Seoul). 2015 Oct;78(4):366-70. doi: 10.4046/trd.2015.78.4.366 [MEDLINE]
- Severe Necrotizing Tracheobronchitis From Panton-Valentine Leukocidin-positive MRSA Pneumonia Complicating Influenza A-H1N1-09. J Bronchology Interv Pulmonol. 2017 Jan;24(1):63-66. doi: 10.1097/LBR.0000000000000314 [MEDLINE]
- Necrotizing tracheobronchitis caused by influenza and Staphylococcus aureus co-infection. Infection. 2018 Oct;46(5):737-739. doi: 10.1007/s15010-018-1180-y [MEDLINE]
Pneumothorax (see Pneumothorax)
- Spontaneous pneumothorax in Asian influenza. S Afr Med J. 1958 Aug 16;32(33):817-8 [MEDLINE]
- Novel influenza A (H1N1) infection: chest CT findings from 21 cases in Seoul, Korea. Clin Radiol. 2011 Feb;66(2):118-24. doi: 10.1016/j.crad.2010.07.011 [MEDLINE]
- Recurrent Spontaneous Pneumothorax during the Recovery Phase of ARDS Due to H1N1 Infection. Balkan Med J. 2013 Mar;30(1):123-5. doi: 10.5152/balkanmedj.2012.086 [MEDLINE]
- Spontaneous Pneumothorax in H1N1 Infection. J Assoc Physicians India. 2017 May;65(5):97-98 [MEDLINE]
Post-Exposure Prophylaxis
- Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med. 2020 Jul 23;383(4):309-320. doi: 10.1056/NEJMoa191534 [MEDLINE]
Treatment
- Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007;45:1568–1575 [MEDLINE]
- Pandemic H1N1 Influenza in Pregnancy Working Group. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010;303:1517–1525 [MEDLINE]
- Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007 Mar 1;44 Suppl 2:S27-72 [MEDLINE]
- Outcomes of adults hospitalised with severe influenza. Thorax 2010;65:510–515 [MEDLINE]
- Fatalities associated with the 2009 H1N1 influenza A virus in New York city. Clin Infect Dis 2010;50:1498–1504 [MEDLINE]
- Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(1):1–24 [MEDLINE]
- Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 2012;55:1198–1204 [MEDLINE]
- A prospective intervention study on higher-dose oseltamivir treatment in adults hospitalized with influenza a and B infections. Clin Infect Dis. 2013 Dec;57(11):1511-9. doi: 10.1093/cid/cit597 [MEDLINE]
- Antivirals for Treatment of Influenza: A Systematic Review and Meta-analysis of Observational Studies. Ann Intern Med. 2012;156:512-524 [MEDLINE]
- Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014 Apr 10;4:CD008965. doi: 10.1002/14651858.CD008965.pub4 [MEDLINE]
- Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545 [MEDLINE]
- Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2547 [MEDLINE]
- Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729 [MEDLINE]
- Impact of outpatient neuraminidase inhibitor treatment in patients infected with influenza A(H1N1)pdm09 at high risk of hospitalization: an individual participant data metaanalysis. Clin Infect Dis 2017;64:1328–1334 [MEDLINE]
- Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis. 2019;68(6):e1 [MEDLINE]
- Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67. doi: 10.1164/rccm.201908-1581ST [MEDLINE]
- Oseltamivir plus usual care versus usual care for influenza-like illness in primary care: an open-label, pragmatic, randomised controlled trial. Lancet. 2020;395(10217):42 [MEDLINE]
- CDC Recommendations for Antiviral Influenza Treatment [CDC]
- Comparison of antiviral agents for seasonal influenza outcomes in healthy adults and children: A systematic review and network meta-analysis. JAMA Netw Open. 2021 Aug 2;4(8):e2119151 [MEDLINE]
Vaccination
- Seasonal influenza vaccination in patients with COPD: a systematic literature review. BMC Pulm Med. 2017 May 3;17(1):79. doi: 10.1186/s12890-017-0420-8 [MEDLINE]
